Pancreatic neuroendocrine tumor susceptibility gene site, and detection method and kit thereof
A neuroendocrine and kit technology, applied in the fields of molecular biology and medicine, can solve the problems of unconfirmed reports on the correlation of YY1 gene with pancreatic neuroendocrine tumors, reports without confirmed correlation with pancreatic neuroendocrine tumors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
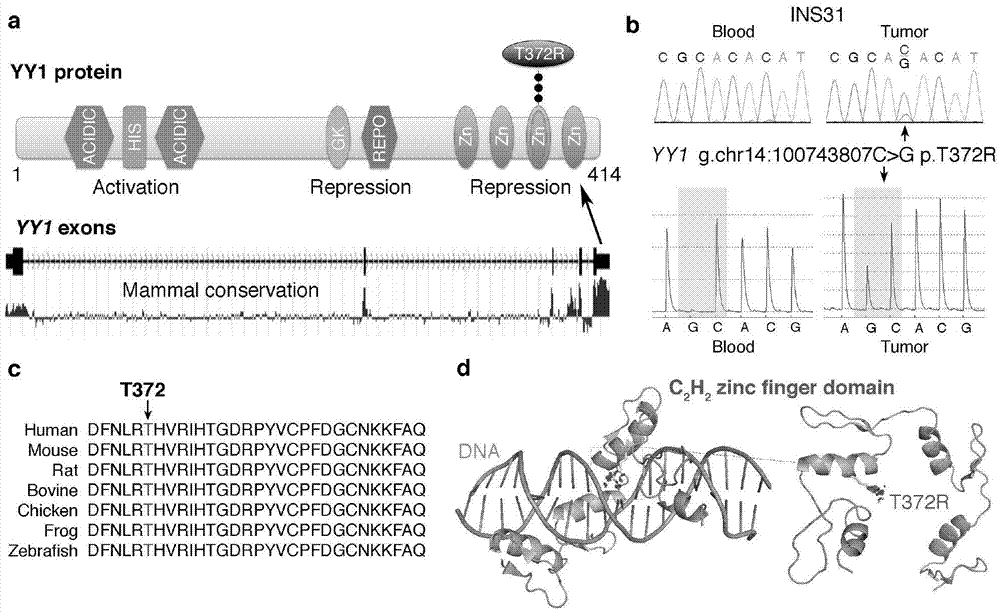
[0099] 1.1 Clinical specimens
[0100] Diagnostic criteria for functional pancreatic neuroendocrine tumors: hypoglycemia and other related clinical symptoms, assessment of blood insulin and blood glucose levels (prolonged oral glucose tolerance test), CT / PET-CT impact on diagnosis, and pathological diagnosis of surgically resected tumors. Genomic DNA was extracted from islet cell tumors preserved in liquid nitrogen and matched peripheral blood samples, and from formalin-fixed paraffin-embedded (FFPE) insulinoma tissue samples. DNA was prepared using the DNeasy kit from QIAGEN and the QIAamp DNA FFPE tissue kit.
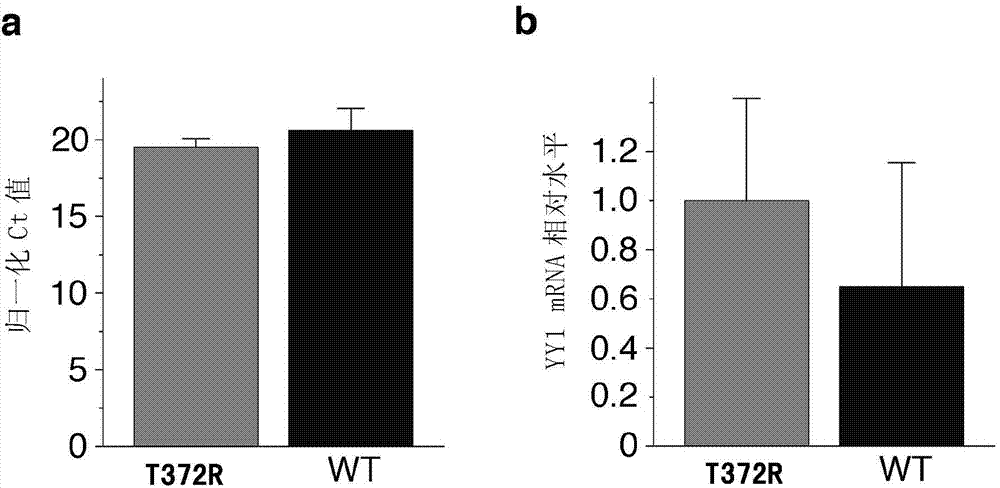
[0101] Deparaffinize FFPE tissue using standard techniques. Immunofluorescence staining was performed on the samples according to standard protocol. The following primary antibodies were used: polyclonal rabbit anti-YY1 antibody (1:100; OriGene, clone EPR4651) and polyclonal guinea pig anti-insulin (1:400, Dako Corporation). Secondary antibodies for immunofluoresce...
Embodiment 2
[0138] Pancreatic Neuroendocrine Tumor Gene Mutation Detection Kit
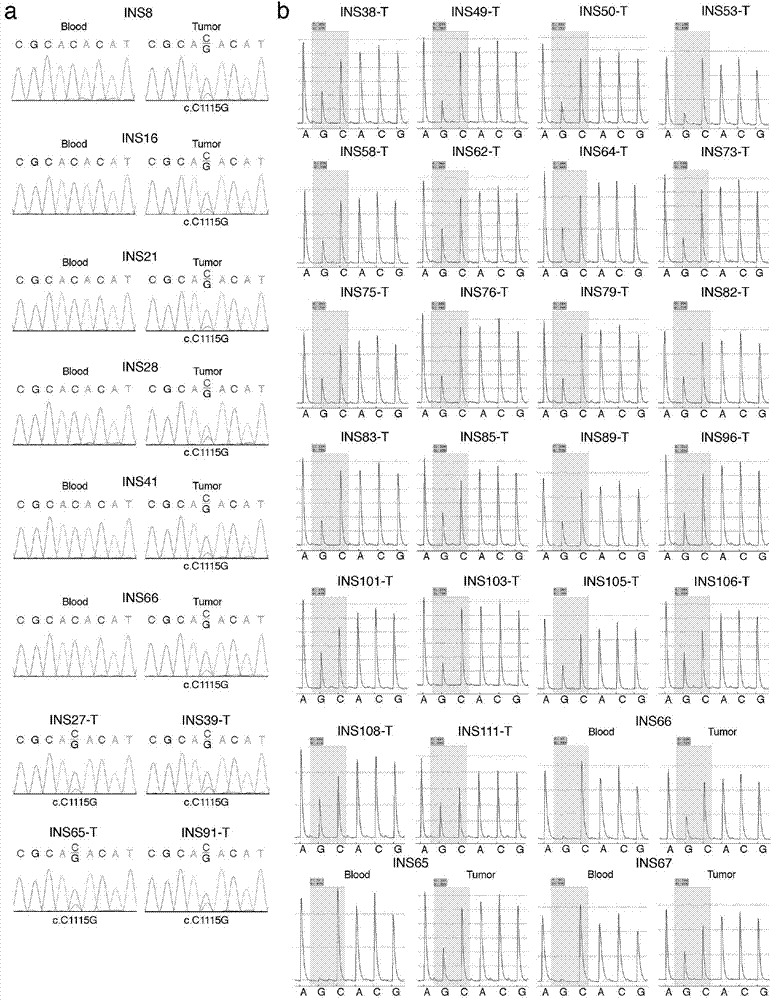
[0139] As described in Example 1, there is a nucleotide sequence of the YY1 gene: that is, the 1115th C→G mutation in SEQ ID NO.: 1 is closely related to pancreatic neuroendocrine tumor diseases. Therefore, specific primers for the YY1 gene can be designed based on this mutation and can be amplified using the patient's DNA as a template for detection.
[0140] Prepare a test kit (100 person-times), which contains:
[0141]
[0142]A test group composed of 100 individuals was randomly selected, including subjects who were unknown whether they had pancreatic neuroendocrine tumors, patients who were known to have pancreatic neuroendocrine tumors, and normal people who were tested to have no pancreatic neuroendocrine tumors.
[0143] Obtain a small amount of islet tissue samples from the subjects to be tested in the test group, and extract DNA using conventional methods. Dilute the PCR primers in the pancrea...
Embodiment 3
[0149] Adjunct detection of pancreatic neuroendocrine tumors
[0150] The test in Example 2 was repeated, with the difference that 80 people (whose symptoms of islet cell tumor were not known before the test) were randomly selected for the test.
[0151] Prepare a test kit (100 person-times), which contains:
[0152]
[0153] Take the islet cell tissue sample of the subject to be tested, and use conventional methods (or use a specific kit) to extract DNA from it. Dilute the PCR primers in the detection kit to 1 μmol / μl, and use the extracted DNA as a template to perform PCR reaction with the provided primers. After the PCR products were purified, they were sequenced with an ABI3730 DNA sequencer, and Polyphred software was used for sequence interpretation and SNV confirmation.
[0154] The results also confirmed that the proportion of islet cell tumors in subjects with C→G mutations at positions 100, 743, and 807 of chromosome 14 (C at position 1115 in SEQ ID NO.: 1) was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com