A kind of external preparation of moxifloxacin hydrochloride and preparation method thereof
A technology of moxifloxacin hydrochloride and ointment, which is applied in the field of external preparations of moxifloxacin hydrochloride, can solve the problems of low osmotic concentration and inability to play a full role, and achieves the effects of rapid penetration and absorption, promotion of wound healing, and promotion of transport.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
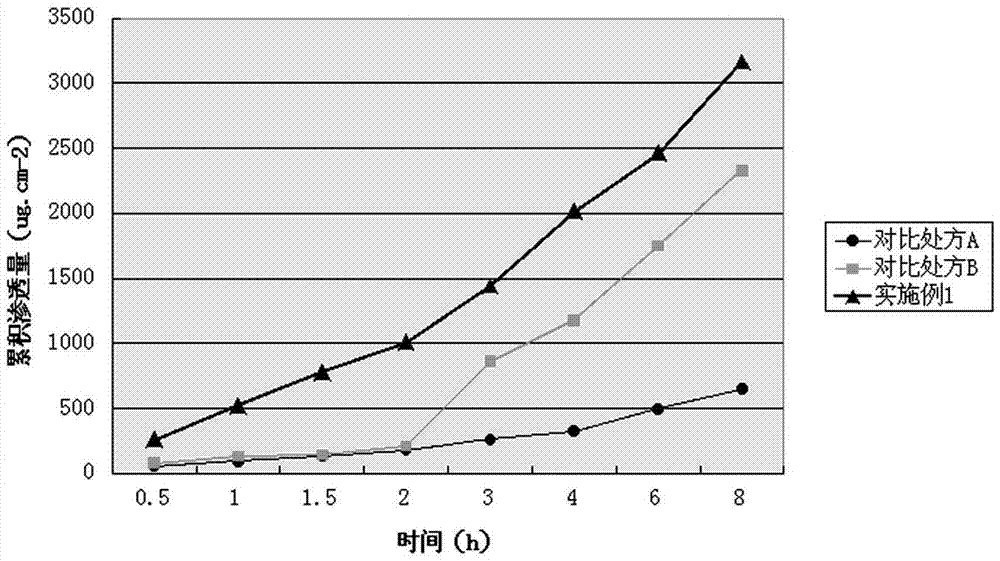
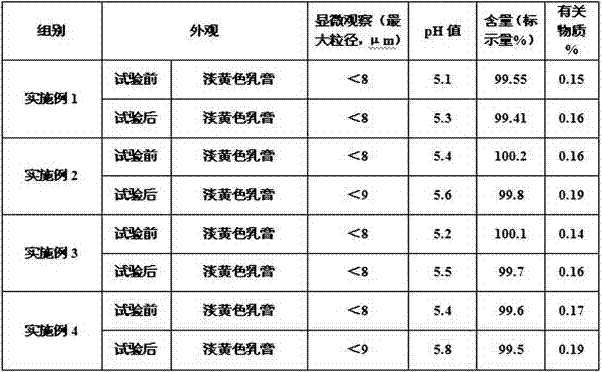
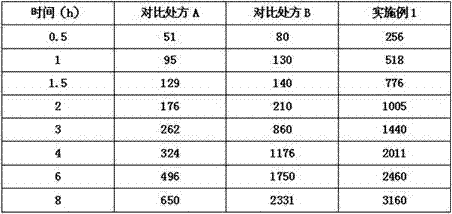
Image
Examples
Embodiment 1
[0041] The prescription is as follows:
[0042] Moxifloxacin Hydrochloride 0.5%
[0043] Stearic Acid 30%
[0044] Glyceryl Monostearate 15%
[0045] White Vaseline 10%
[0046] Lanolin 12%
[0047] Glycerin 8%
[0048] Ethylparaben 0.06%
[0049] Edetate Disodium 0.05%
[0050] Triethanolamine 4%
[0051] N-Trimethyl Chitosan 3%
[0052] Menthol 2%
[0053] Isopropyl myristate 1%
[0054] Purified water balance.
[0055] Preparation method: The following materials are fed according to the prescription quantity
[0056] 1) Heat stearic acid, glyceryl monostearate, lanolin, and white petrolatum to 75-80°C and mix well to obtain the oil phase;
[0057] 2) First put N-trimethyl chitosan into 75% of the prescription amount of purified water, stir until it is completely dissolved, then add menthol, after stirring evenly, add disodium edetate, ethylparaben, and triethanolamine in sequence , heated to 75-80°C and stirred evenly to obtain an aqueous phase;
[0058] 3) Ad...
Embodiment 2
[0061] The prescription is as follows:
[0062] Moxifloxacin Hydrochloride 0.8%
[0063] Stearic Acid 20%
[0064] Glyceryl Monostearate 10%
[0065] White Vaseline 8%
[0066] Lanolin 10%
[0067] Glycerin 6%
[0068] Chlorobutanol 0.04%
[0069] Edetate Disodium 0.05%
[0070] Triethanolamine 3%
[0071] N-Trimethyl Chitosan 3%
[0072] Menthol 2%
[0073] Isopropyl myristate 1%
[0074] Purified water balance.
[0075] Preparation method: The following materials are fed according to the prescription quantity
[0076] 1) Heat stearic acid, glyceryl monostearate, lanolin, and white petrolatum to 75-80°C and mix well to obtain the oil phase;
[0077] 2) First put N-trimethyl chitosan into 75% of the prescription amount of purified water, stir until completely dissolved, then add menthol, stir well, then add disodium edetate, chlorobutanol, triethanolamine in turn , heated to 75-80°C and stirred evenly to obtain an aqueous phase;
[0078] 3) Add the water phase to...
Embodiment 3
[0081] The prescription is as follows:
[0082] Moxifloxacin Hydrochloride 0.5%
[0083] Butyl flufenamate 0.1%
[0084] Stearic Acid 20%
[0085] Glyceryl Monostearate 10%
[0086] White Vaseline 8%
[0087] Lanolin 10%
[0088] Glycerin 6%
[0089] Ethylparaben 0.04%
[0090] Edetate Disodium 0.05%
[0091] Triethanolamine 3%
[0092] N-Trimethyl Chitosan 3%
[0093] Menthol 2%
[0094] Isopropyl myristate 1%
[0095] Purified water balance.
[0096] Preparation method: The following materials are fed according to the prescription quantity
[0097] 1) Heat stearic acid, glyceryl monostearate, lanolin, and white petrolatum to 75-80°C and mix well to obtain the oil phase;
[0098] 2) First put N-trimethyl chitosan into 75% of the prescription amount of purified water, stir until it is completely dissolved, then add menthol, after stirring evenly, add disodium edetate, ethylparaben, and triethanolamine in sequence , heated to 75-80°C and stirred evenly to obtain a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com