Alpha, alpha-dimethylbenzyl alcohol hydrogenolysis method for preparing isopropyl benzene
A technology of dimethyl benzyl alcohol and cumene, applied in the field of α, which can solve the problems of unstable catalyst, large amount of precious metal, and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
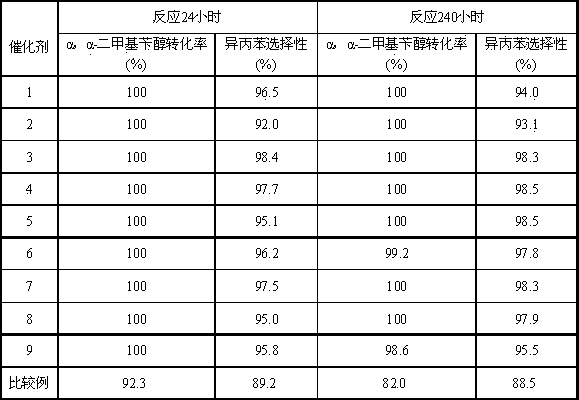
[0023] Combine 100 g silica with 100 g Mg 2+ Impregnated in equal volume of aqueous solution with a content of 2%, dried, 600 o After calcination of C for 4 h, MgO-modified precursor I was obtained; 1.0 g of PdCl 2 , 15.0 g Ni (NO 3 ) 2 ·6H 2 O, solution I was obtained in a solution consisting of 10.0 g concentrated hydrochloric acid and 74.0 g purified water. 50g of solution I was dipped on 50g of precursor I, dried at 500 o Catalyst 1 was obtained by calcining at C for 4.0 h. Its composition is: 0.57 parts of Pd-3.09 parts of MgO-3.68 parts of NiO-92.67 parts of SiO 2 .
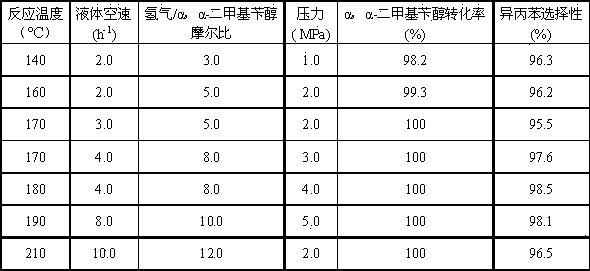
[0024] Load 40.0 ml of catalyst 1 into a fixed bed reactor, 250 o Hydrogen reduction at C for 4 hours. The α,α-dimethylbenzyl alcohol-containing hydrocarbon material contains 15% α,α-dimethylbenzyl alcohol and 85% cumene by weight percentage. Reaction process condition is: reaction inlet temperature 170 ℃, reaction pressure 2.0 MPa, H 2 / α,α-dimethylbenzyl alcohol molar ratio 8.0, liquid volume s...
Embodiment 2
[0027] Combine 100 g silica with 100 g Ca 2+ Impregnated in equal volume of aqueous solution with a content of 2%, dried, 500 o After calcination of C for 4 h, the CaO-modified precursor I was obtained; 1.5 g of PdCl2 , 15.0 g Ni (NO 3 ) 2 ·6H 2 O, solution I was obtained in a solution composed of 10.0 g concentrated hydrochloric acid and 73.5 g purified water. 50g of solution I was dipped on 50g of precursor I, dried at 500 o Catalyst 2 was obtained by calcining at C for 4.0 h. Its composition is: 0.85 parts of Pd-2.6 parts of CaO-3.67 parts of NiO-92.87 parts of SiO 2 .
[0028] Load 40.0 ml of catalyst 2 into a fixed bed reactor, 250 o Hydrogen reduction at C for 4 hours. The hydrocarbon material containing α,α-dimethylbenzyl alcohol contains 25% α,α-dimethylbenzyl alcohol and 75% cumene by weight percentage. Reaction process conditions are: reaction inlet temperature 170 ℃, reaction pressure 3.0 MPa, H 2 Molar ratio of / α,α-dimethylbenzyl alcohol 10.0, liquid vol...
Embodiment 3
[0031] Combine 100 g silica with 100 g Ba 2+ Impregnated in equal volume of aqueous solution with a content of 2%, dried, 500 o BaO-modified precursor I was obtained after calcination of C for 4 h; 1.5 g of PdCl 2 , 15.0 g Ni (NO 3 ) 2 ·6H 2 O, solution I was obtained in a solution composed of 10.0 g concentrated hydrochloric acid and 73.5 g purified water. 50g of solution I was dipped on 50g of precursor I, dried at 500 o Catalyst 3 was obtained by calcining at C for 4.0 h. Its composition is: 0.85 parts of Pd-2.09 parts of BaO-3.67 parts of NiO-93.39 parts of SiO 2 .
[0032] Charge 40.0 ml of catalyst 3 into a fixed bed reactor, 250 o Hydrogen reduction at C for 6 hours. The hydrocarbon material containing α,α-dimethylbenzyl alcohol contains 25% α,α-dimethylbenzyl alcohol and 75% cumene by weight percentage. Reaction process conditions are: reaction inlet temperature 170 ℃, reaction pressure 3.0 MPa, H 2 Molar ratio of / α,α-dimethylbenzyl alcohol 10.0, liquid vol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com