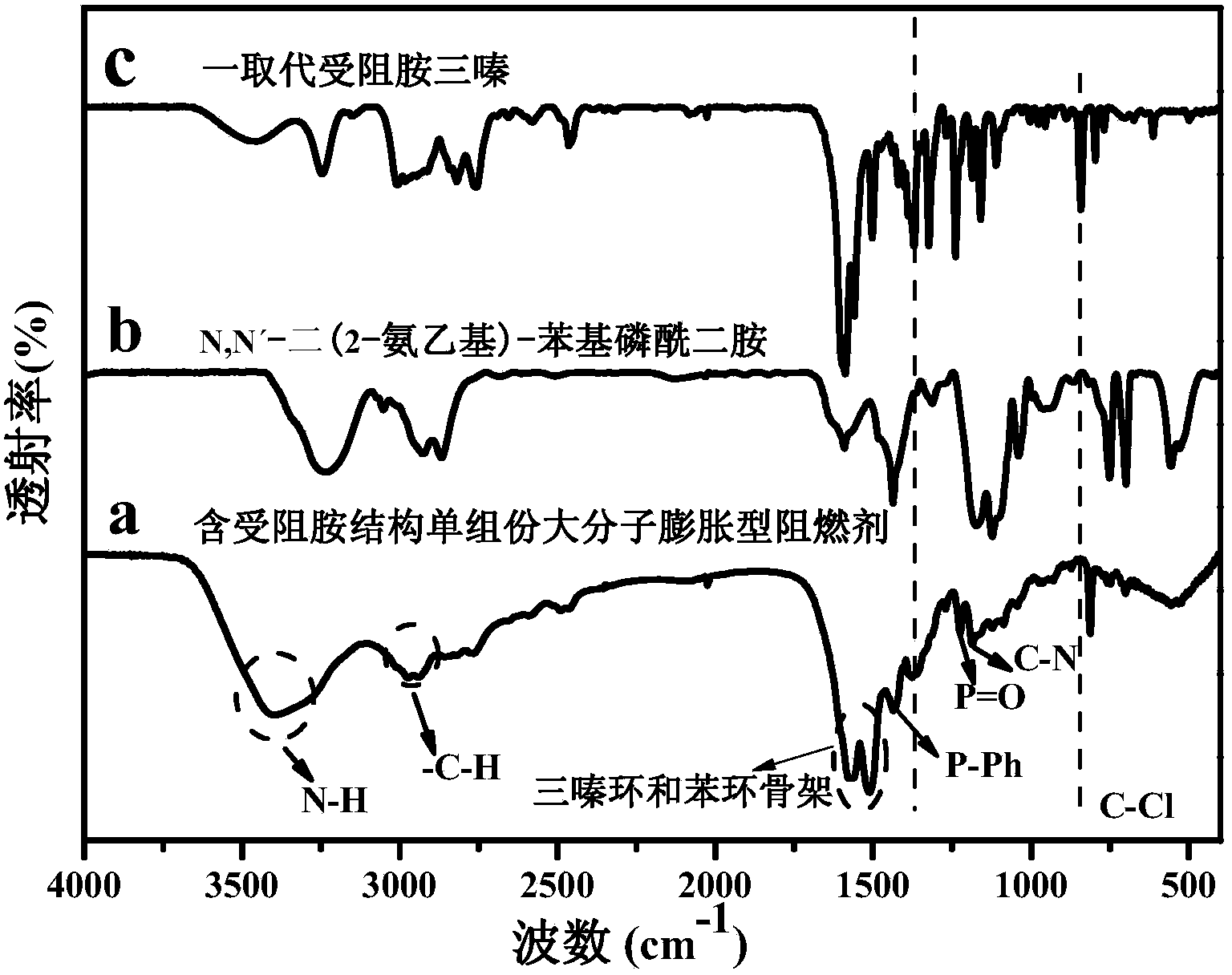
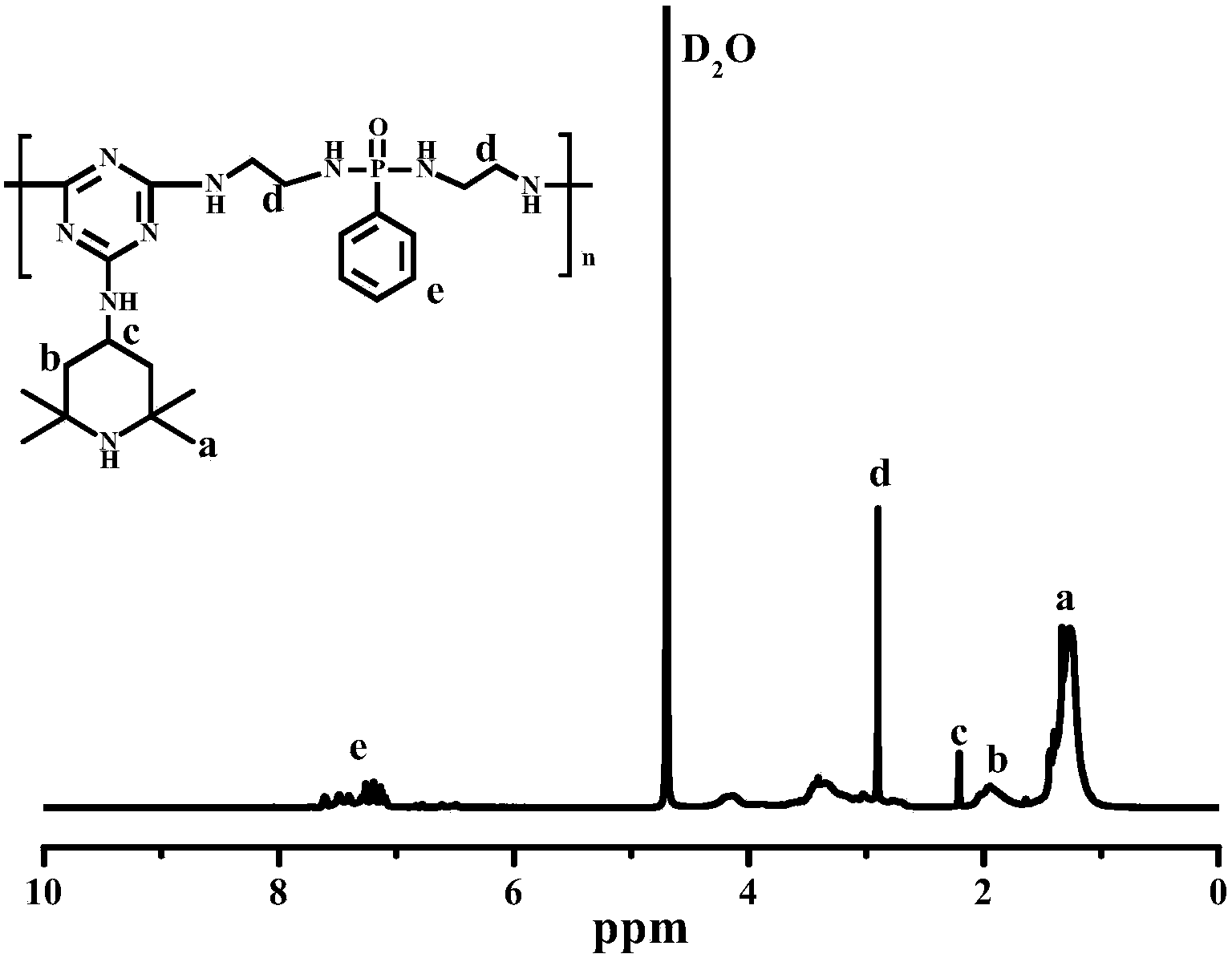
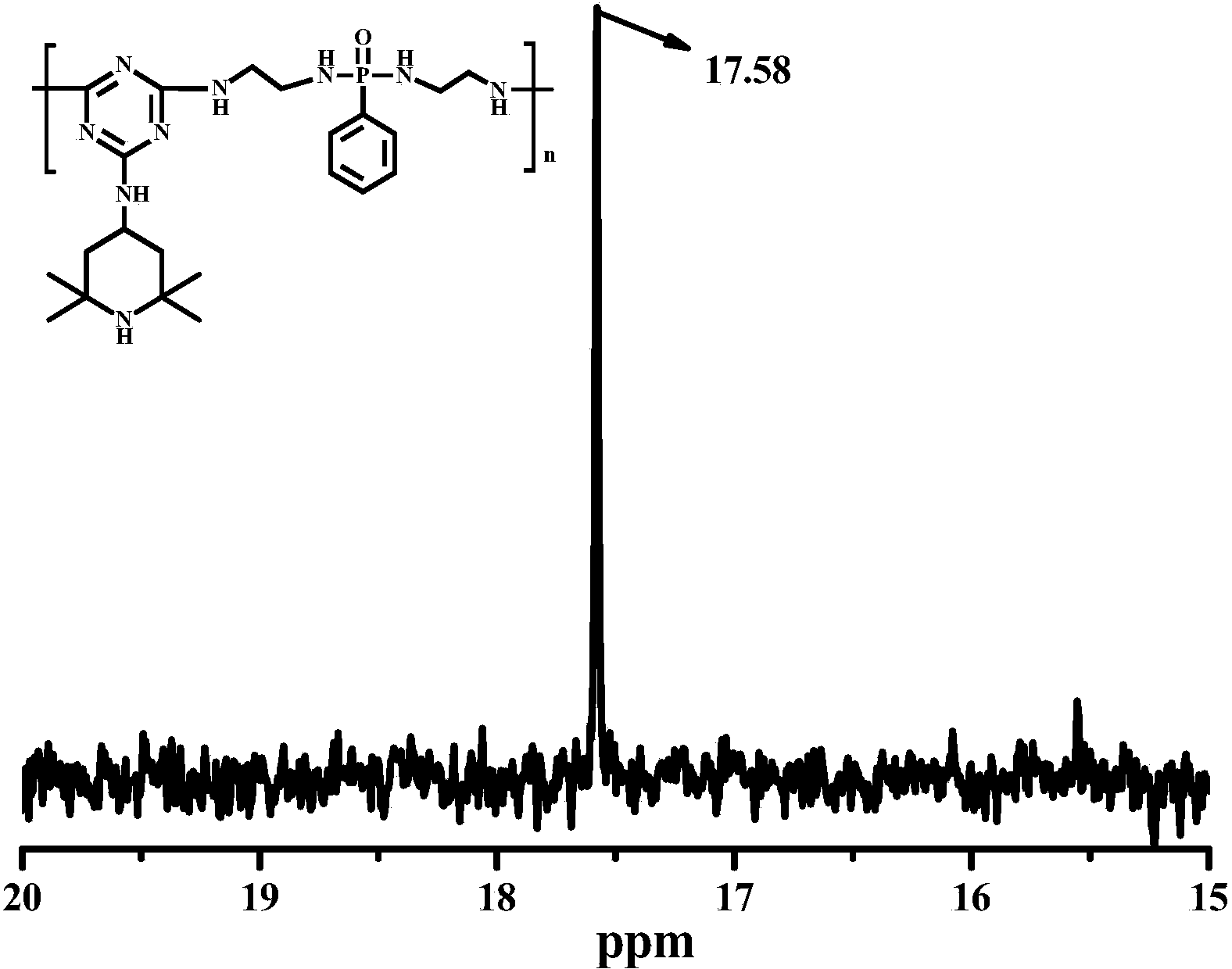
Macromolecular expansion type flame retardant containing hindered amine structure and preparation method and application of macromolecular expansion type flame retardant
A technology of intumescent flame retardants and hindered amines, which is applied in the preparation and application of halogen-free flame retardants, can solve the problems of easy migration, poor thermal stability, and poor compatibility of small molecule intumescent flame retardants, and achieve production High efficiency, improved weather resistance, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1) Synthesis of a substituted hindered amine triazine compound: Add 250 mL of toluene and 36.88 g (0.2 mol) of cyanuric chloride into a 500 ml four-neck flask under ice bath conditions at 0°C to 5°C, and stir at a constant speed for 30 minutes; g (0.2mol) 2,2,6,6-tetramethylpiperidinamine was dissolved in 50mL of toluene, and was added dropwise to the reaction kettle within 3 hours, while slowly adding a 20wt% sodium hydroxide aqueous solution, the dropwise After the reaction for 3 hours; after the reaction, the mixture was concentrated, filtered, washed with deionized water and ethanol successively, and then dried to obtain a substituted hindered amine triazine compound.
[0045] 2) Synthesis of a single-component macromolecule intumescent flame retardant containing a hindered amine structure: add 300 mL of dioxane and 45.62 g (0.15 mol) of a substituted hindered amine triazine compound into a 500 mL four-neck flask, and heat up to 55 °C (oil bath), stir at a constant ...
Embodiment 2
[0050] The differences between this example and Example 1 are: the amount of polypropylene in step 3) is changed to 75wt%, and the amount of one-component macromolecule intumescent flame retardant containing hindered amine structure is changed to 25wt%. Wherein the n in the structural formula is 20, and the molecular weight is 10000
[0051] The test results of flame retardancy, mechanical properties, water resistance and weather resistance are shown in Table 1 and Table 2.
Embodiment 3
[0053] The differences between this example and Example 1 are: the amount of polypropylene in step 3) is changed to 70wt%, and the amount of one-component macromolecular intumescent flame retardant containing hindered amine structure is changed to 30wt%. Wherein the n in the structural formula is 20, and the molecular weight is 10000
[0054] The test results of flame retardancy, mechanical properties, water resistance and weather resistance are shown in Table 1 and Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Impact strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com