In-vitro culture method of balantidium coli
A technology for in vitro culture and ciliates, applied in microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of poor stability and complicated preparation, and achieve the effects of good stability, simple preparation method and convenient operation.
Inactive Publication Date: 2015-01-21
HENAN UNIV OF SCI & TECH
View PDF3 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The culture media such as LE, Robinson's, TYSGM-9, Balamuth's, Jones's, etc. currently used for in vitro culture of schizociliates coli have problems such as complex preparation and poor stability (Clark, C.G., and L.S. Diamond. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 2002, 15:329–341.)
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
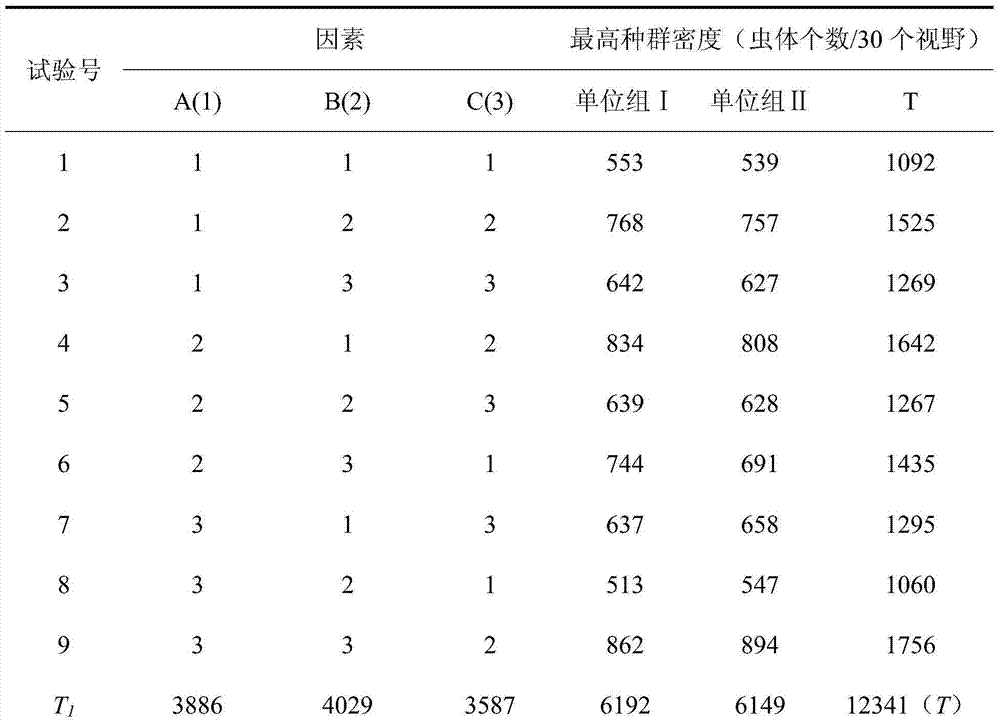
[0022] This embodiment provides an in vitro culture method of schizociliates colon, the steps are as follows:
[0023] 1. Pest inoculation:
[0024] Inoculate the porcine feces containing paricociliasis into the RSS medium, and culture at 28°C for 48h.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses an in-vitro culture method of balantidium coli. The in-vitro culture method of balantidium coli comprises the following steps of (1) polypide inoculation: inoculating pollutant containing balantidium coli in an RSS culture medium and culturing for 48 h at a temperature of 28 DEG C; (2) preparation of the culture medium: preparing the culture medium according to 15-25% by volume of new-born calf serum and 75-85% by volume of a 199 culture medium with a pH value of 6.0-8.0, adding 20-40 mg of starch in every 3 mL of the culture medium; and (3) polypide culture: (a) adding 10,000 U / mL of penicillin and streptomycin in the culture medium formed by the step (2), (b) inoculating a balantidium coli suspension in the culture medium formed by the step (a), culturing for 96-108 h at the temperature of 28 DEG C to obtain balantidium coli having the highest population density being 42.75-74.5 times of an inoculation density. The in-vitro culture method of balantidium coli has the advantages of simple preparation method, convenient operations, relatively good stability and high population density.
Description
technical field [0001] The invention relates to an in vitro culture method of schizociliates colon, belonging to the technical fields of animal parasitology and protozoan in vitro culture. Background technique [0002] Balantidium coli is a protozoan parasitic in the intestinal tract and is currently known to be the largest protozoa infecting humans and non-human primates (Solaymani-Mohammadi S, Petri WA Jr. Zoonotic implications of swine-transmitted protozoal infections. Vet Parasitol 2006, 140:189–203). Balantidiosis is a worldwide distribution, mainly prevalent in tropical and subtropical regions. Human balanticiliasis is acquired through contaminated water sources, vector insects, sanitary conditions (including personal and environmental), and close contact with pigs. The infection rate of immunosuppressed patients such as AIDS patients and leukemia patients is higher than that of normal people; the infection rate of people in mental hospitals and shelters is higher th...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C12N1/10C12R1/90
CPCC12N1/10
Inventor 王天奇闫文朝钱伟锋丁轲韩利方
Owner HENAN UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com