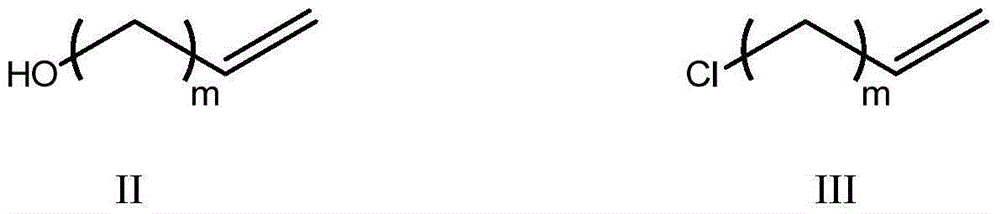Omega-vinylsulphonyl disulfide compound as well as preparation method and application thereof
A sulfur compound and vinyl sulfone technology, which is applied in the fields of ω-vinylsulfone-based disulfide compound, its preparation and application, and achieves the effects of simple and easy method, broad-spectrum reaction and convenient application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
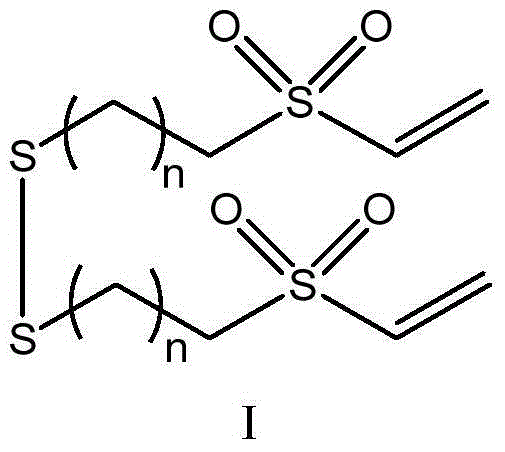
[0027] The invention provides an ω-vinylsulfone-based disulfide compound, its preparation method and application. The ω-vinylsulfone-based disulfide compound of the present invention has the structure of general formula I:
[0028]
[0029] In the general formula I, n is an integer of 4-18; n is preferably an integer of 8-12; more preferably an integer of 9-11.
[0030] The preparation method of the ω-vinylsulfone group disulfide compound provided by the present invention comprises the following steps:
[0031] (1) the compound of formula II and triphenylphosphine molar ratio 1:1.2~1.4 were refluxed in carbon tetrachloride 4 hours and prepared the compound of formula III;
[0032]
[0033] Among them, m=n-1;
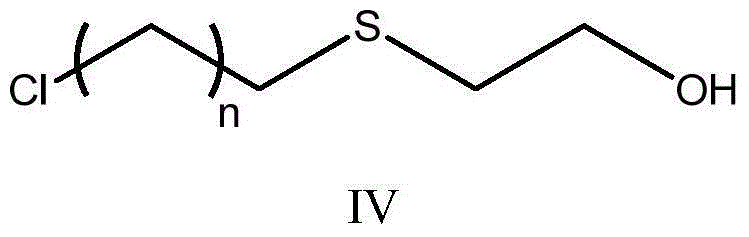
[0034] (2) the compound of formula III, azobisisobutyronitrile (AIBN) and mercaptoethanol in a molar ratio of 1:0.55:1.5~4 were reacted for 3 hours under the conditions of 70~90°C in petroleum ether to prepare the compound of formula IV;
[0035]
[0036] (3)...
Embodiment 1
[0057] The preparation (n=10) of compound i comprises the following steps:
[0058] (1) Compound ii and triphenylphosphine were refluxed in carbon tetrachloride for 4 hours at a molar ratio of 1:1.2 to prepare compound iii, 94.6%. 1 H NMR (400MHz, CDCl 3 ):δ5.80(m,1H,CH=CH 2 ),4.97(dd,2H,J 1 =16.8,J 2 =10.0, CH=CH 2 ), 3.53(t, 2H, J=6.8, ClCH 2 ),2.03(m,2H,CH 2 CH=CH 2 ),1.77(m,2H,ClCH 2 CH 2 ),1.28~1.44(m,12H, others CH 2 ). 13 C NMR (100MHz, CDCl 3 ): δ139.2 (CH=CH 2 ), 114.1 (CH=CH 2 ), 45.2 (ClCH 2 ), 33.8 (CH 2 =CHCH 2 ), 32.7 (ClCH 2 CH 2 ), 26.9 (ClCH 2 CH 2 CH 2 ),28.9~29.4(other CH 2 ).MS(EI + ):m / z[M] + 188.13.
[0059]
[0060] (2) Compound iii was reacted with azobisisobutyronitrile (AIBN) and mercaptoethanol at a molar ratio of 1:0.55:1.5 in petroleum ether at 84°C for 3 hours to prepare compound iv with a yield of 88.2%. 1 H NMR (400MHz, CDCl 3 ,): δ3.72(t,2H,J=6.0,CH 2 OH), 3.53(t, 2H, J=6.8, ClCH 2 ),2.73(t,2H,J=6.0,SCH 2 CH 2 ...
Embodiment 2
[0069] Preparation and Characterization of Self-Assembled Films
[0070] (1) Prepare a self-assembled film using the compound i prepared in Example 1 as a self-assembled material:
[0071] a. Preparing the mother liquor: using dichloromethane and ethanol as a solvent in a volume ratio of 1:1, fully dissolving compound i, and preparing a self-assembly mother liquor with a concentration of 1 mmol;
[0072] b. Soak the gold chip in the self-assembly mother solution prepared in step a, and let it stand at 25±2°C for 24 hours;
[0073] c. The resulting self-assembled membrane was rinsed with ethanol and stored in the dark.
[0074] (2) The XPS characterization results of the self-assembled membrane prepared in step (1) are as follows
[0075]
[0076] 1Binding Energy=84.2eV
[0077] 2 Binding Energy = 162.0eV
[0078] 3 Binding Energy = 168.4eV
[0079] 4Binding Energy=285.0eV
[0080] 5 Binding Energy = 286.2eV
[0081] 6Binding Energy=531.8eV
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com