siRNA aiming at human annexin A2 acceptor gene and application thereof
A human annexin and receptor technology, applied in the field of genetic engineering, can solve the problems of short duration and multiple treatments, and achieve the effect of inhibiting proliferation and inducing block.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Synthesis of siRNA
[0027] Search NCBI GeneBank to obtain the full sequence of AXIIR and mRNA sequence, use the existing network resources and commonly used software for biological analysis of AXIIR, and select the coding region as the target sequence for siRNA design. Refer to the siRNA design principle, and compare it with the human genome sequence through the blast function of the GeneBank database to ensure that there is no homology; exclude potential siRNAs that have 8 consecutive bases at the 5' end of the aitisense chain paired with other genes; exclude any consecutive 14 bases base-pairing potential siRNAs with other genes.
[0028] The siRNA sequence designed and synthesized in this embodiment is
[0029]
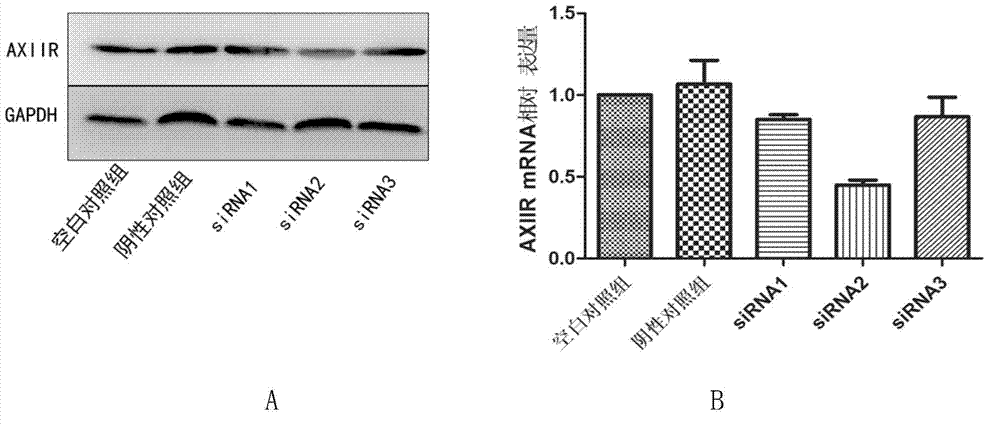
[0030] From figure 1 It can be seen that siRNA2 can inhibit the expression of AXIIR by about 20%; siRNA2 can inhibit the expression of AXIIR to more than 50%, and siRNA3 can inhibit the expression of AXIIR by about 20%.
[0031] Therefore, ...
Embodiment 2
[0032] Example 2: Cell Transfection
[0033] (1) One day before transfection, collect the cells in the logarithmic growth phase and inoculate them in a 12-well plate, and the inoculated number is about 5×10 4 cells, add 1 mL of culture medium.
[0034] (2) Add 4 μL liposome transfection reagent to 100 μL Opti-MEM medium, pipette gently, and let stand at room temperature for 5 minutes.
[0035] (3) Add 60Nm to 100μL Opti-MEM medium and mix gently.
[0036](4) Mix the transfection reagent and the siRNA diluent, blow evenly, and let stand at room temperature for 20 minutes.
[0037] (5) Change the medium 8 hours after transfection, and continue to cultivate for 48 hours before performing other operations after transfection.
Embodiment 3
[0038] Example 3: Detection of mRNA and protein expression of AXIIR in umbilical vein endothelial cells by real-time fluorescent quantitative RT-PCR and western blotting
[0039] The specific process is as follows:
[0040] 1 Real-time fluorescent quantitative RT-PCR
[0041] (1) Extraction of total RNA: Collect cells into a 1.5 mL RNase-free centrifuge tube, add 0.5 mL Trizol, mix well on ice and pipette, and let stand for 10 min. Add 0.125mL chloroform, shake vigorously for 20s, and let stand on ice for 5min. Centrifuge at 4°C, 12000r / min×15min, pipette 0.2mL supernatant to another 1.5mL, then add the same amount of isopropanol as the supernatant, mix gently, and let stand on ice for 10min. Centrifuge at 4°C, 12000r / min×15min, discard the supernatant, add 1mL pre-cooled 75% ethanol, gently wash the precipitate, centrifuge at 4°C, 12000r / min×15min. Discard the supernatant, dry it, and dissolve it in 20 μL DEPC water. A multifunctional microplate reader was used to determi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com