Compositions comprising zoledronic acid or related compounds for relieving inflammatory pain and related conditions
A technology for zoledronic acid and inflammatory pain, which can be used in drug combinations, non-central analgesics, anti-inflammatory agents, etc., and can solve problems such as low oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
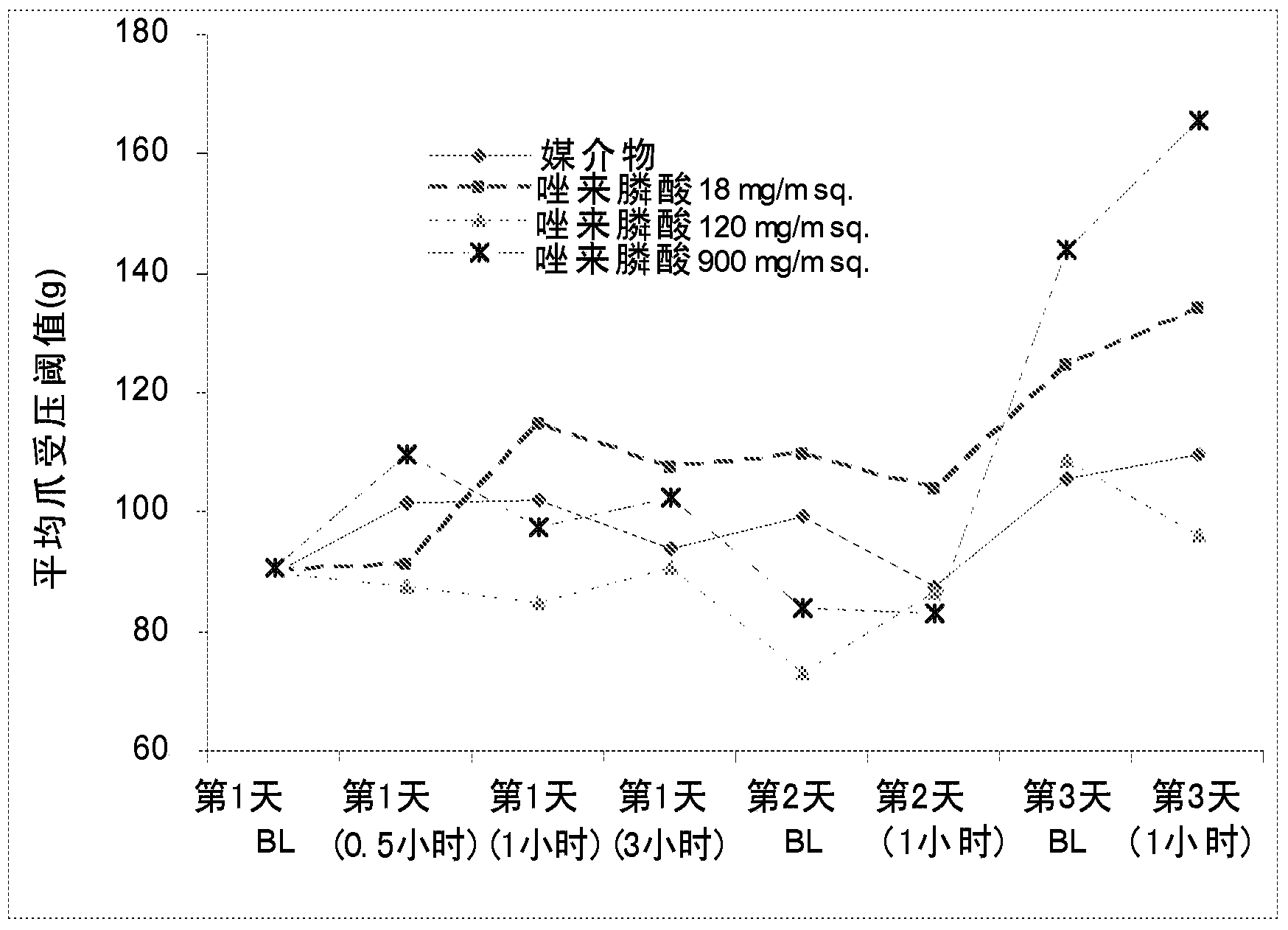
[0088] Effects of Orally Administered Zoledronic Acid in a Rat Model of Inflammatory Pain
[0089] method:
[0090] The effect of orally administered zoledronic acid on inflammatory pain was examined using the complete Freund's adjuvant (CFA) model in the rat. Inflammatory pain was induced by injecting a volume of 75 [mu]L of 100% CFA into the left hind paw of Sprague-Dawley rats on day 0 and then assessed on days 1-3. Animals were orally administered vehicle (control), zoledronic acid 18 mg / m once daily on days 1-3 2 (or 3mg / kg), zoledronic acid 120mg / m 2 (or 20mg / kg) or zoledronic acid 900mg / m 2 (or 150mg / kg). Drugs were dissolved in distilled water and prepared fresh daily. Animals were fasted prior to dosing. Under current FDA guidance on extrapolating starting doses from animals to humans, it is considered 2 The indicated doses are equivalent across mammalian species. Thus, for example, 18 mg / m in rats is considered 2 Equivalent to 18mg / m in humans 2 , while 3...
Embodiment 2
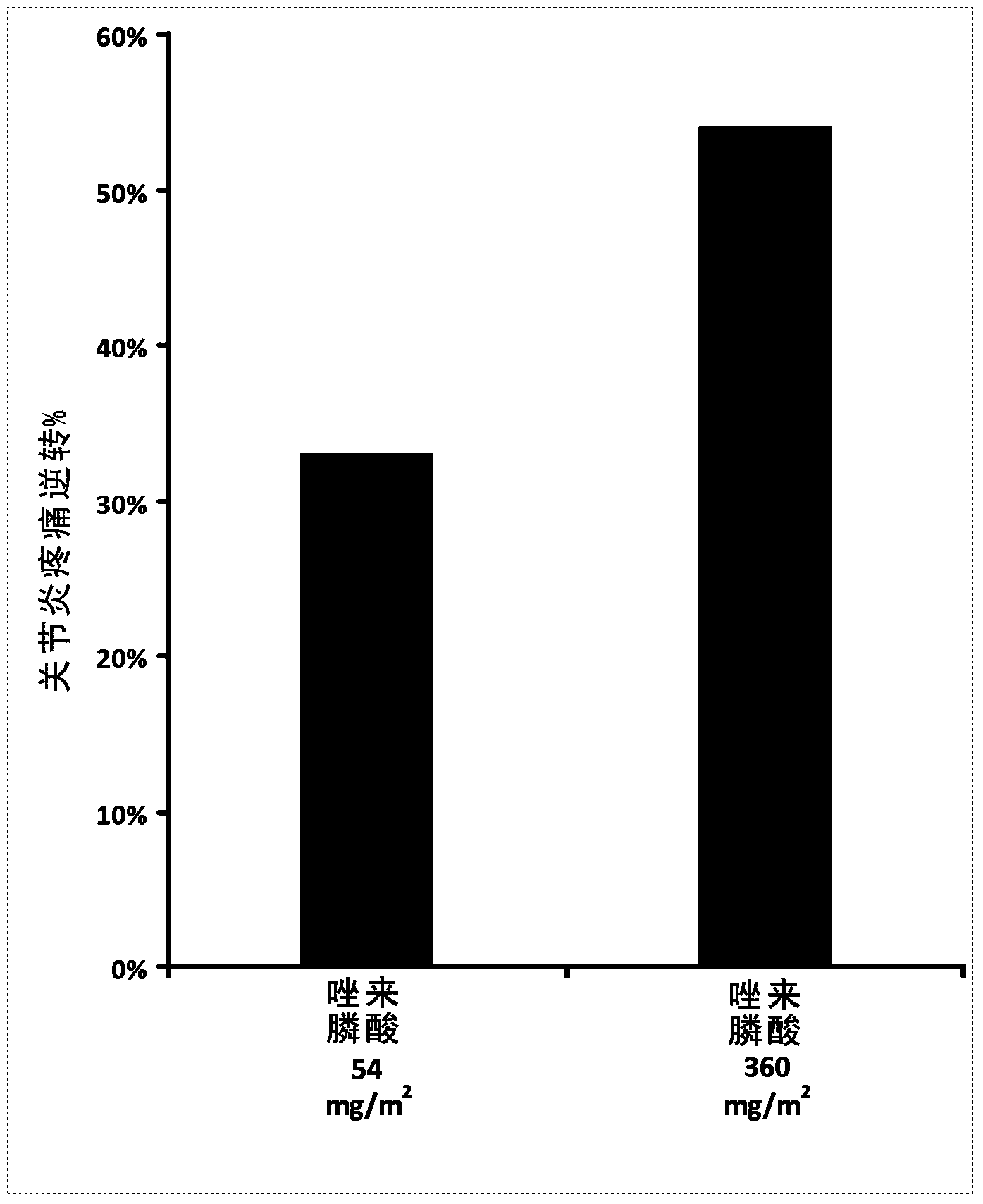
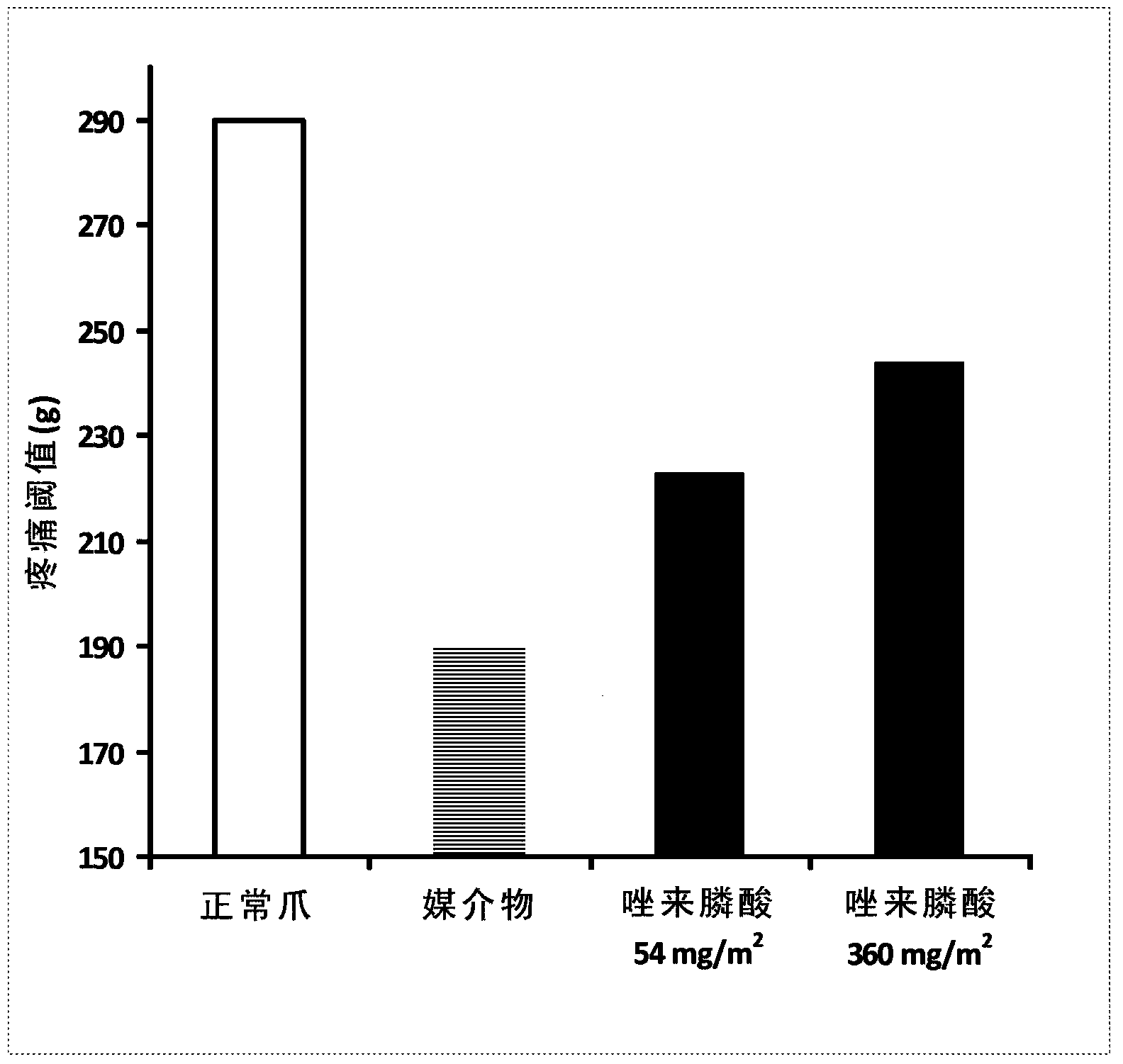
[0100] Effects of Orally Administered Zoledronic Acid in a Rat Model of Arthritis Pain
[0101] method:
[0102] The effect of orally administered zoledronic acid on arthritic pain was examined in the rat Complete Freund's Adjuvant (CFA) model of arthritic pain. In this model, 100% complete Freund's adjuvant (CFA) in a volume of 75 μL was injected into the left hind paw, followed by the development of arthritic pain over a period of 10-14 days. During the first three days after CFA injection, animals were orally administered three equal daily doses of vehicle (control), zoledronic acid 54 mg / m 2 (or 9mg / kg) or zoledronic acid 360mg / m 2 (or 60mg / kg). Drugs were dissolved in distilled water and prepared fresh daily. Animals were fasted prior to dosing.
[0103] Arthritic pain (mechanical hyperalgesia) was assessed in vehicle and drug-treated animals on day 14 after CFA injection using the Randall-Selitto digital device (dRS; IITC Life Sciences, Woodland Hills, CA). Anima...
Embodiment 3
[0109] Example 3 Treatment of complex regional pain syndrome with orally administered zoledronic acid.
[0110]The effect of orally administered zoledronic acid was examined in the rat tibial fracture model of complex regional pain syndrome (CRPS). CRPS in rats was induced by fracture of the right distal tibia of the animals and casting of the fractured hind paw for 4 weeks as described by Guo TZ et al. (Pain. 2004; 108:95-107) stated. This animal model has been shown to reproduce the stimulus trauma, natural history, signs, symptoms and lesions observed in human CRPS patients (Kingery WS et al., Pain. 2003; 104:75-84).
[0111] From the day of fracture and injection, animals were treated with 18mg / m 2 Doses per day (3 mg / kg / day) were administered orally with vehicle (control) or zoledronic acid for 28 days. Drugs were dissolved in distilled water and administered by gavage. Animals were fasted 4 hours before and 2 hours after dosing. At the end of the 28 day period, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Body surface area | aaaaa | aaaaa |
| Body surface area | aaaaa | aaaaa |
| Body surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com