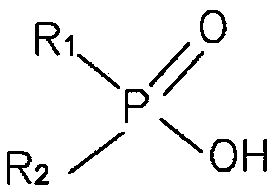
Preparation method of dialkyl phosphinic acid
A technology of dihydrocarbyl phosphinic acid and halogenated hydrocarbons, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problem of many reaction steps, small steric hindrance and complex process and other problems, to achieve the effects of reducing production costs, mild reaction conditions, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
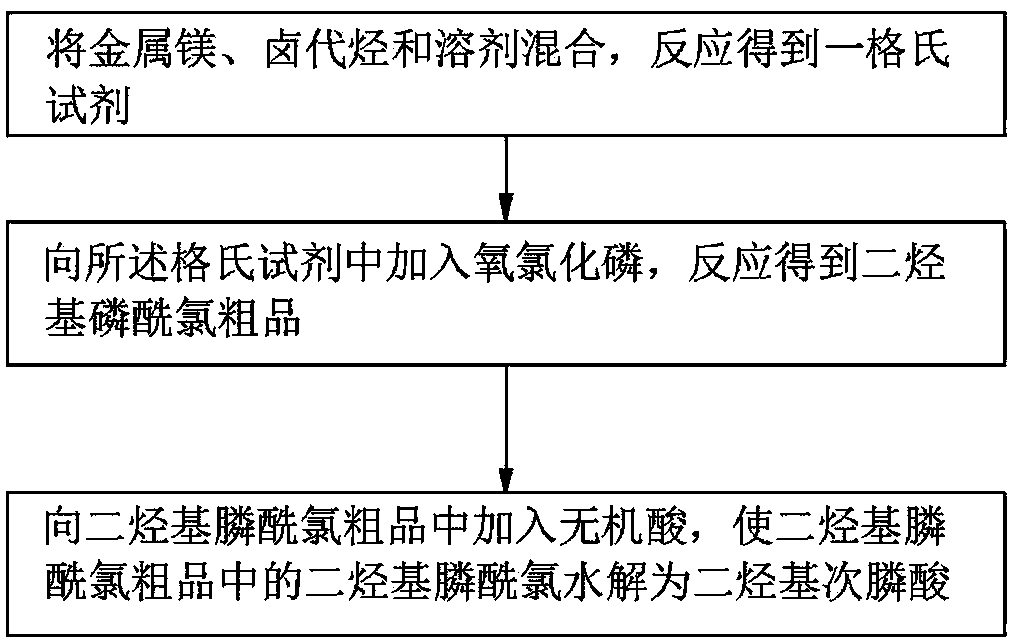
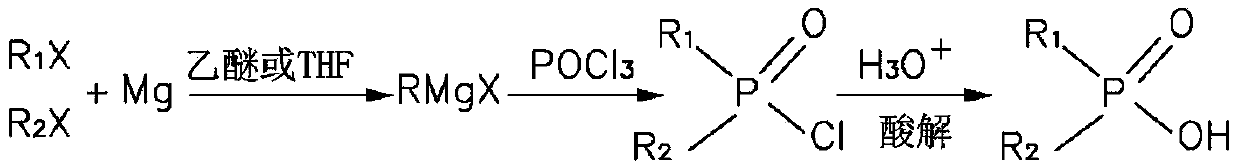
[0017] Please also refer to figure 2 as well as image 3 , the preparation method of the dihydrocarbyl phosphinic acid provided by the present invention, the preparation method comprises the following steps:
[0018] Step 1, metal magnesium, halogenated hydrocarbon and solvent are mixed and reacted to obtain a Grignard reagent.
[0019] The Grignard reagent refers to the reaction of organic halogen compounds (halogenated hydrocarbons, active halogenated aromatic hydrocarbons) and metal magnesium in a solvent to form an organic magnesium reagent. The process is specifically:
[0020] Put a certain amount of metal magnesium bars into a three-necked flask with stirring, reflux and drying devices, then add a small amount of solvent, just to immerse the metal magnesium bars, and then add a small amount of mixture of halogenated hydrocarbons and solvents to initiate the reaction, heat Until the system obviously reacts, continue to add the above mixed solution dropwise after a fe...
Embodiment 1
[0037] Put 2.16g metal magnesium strips (0.09mol) into a three-necked flask equipped with a stirring, reflux, drying device and a constant pressure dropping funnel, and add 5mL of ether to immerse the magnesium strips. Mix 11.6g of brominated isooctane (0.06mol) with 30mL of diethyl ether and place in a constant pressure dropping funnel. First add about 1 / 10 volume of the above mixed solution into the flask, and heat until the system obviously reacts. After 10 minutes, under the state of maintaining reflux, continue to slowly add the rest of the above mixed solution dropwise. After the dropwise addition, maintain reflux and react for about 2 hours. After the reaction, the system was cooled to room temperature. 3.67g POCl 3 (0.024mol) mixed with 10ml of diethyl ether, placed in a constant pressure dropping funnel and slowly added dropwise to the above reaction system, and continued to stir for about 1 hour after the dropwise addition was completed. Then add 60 mL of H with a...
Embodiment 2
[0040] Put 2.16g metal magnesium bars (0.09mol) into a three-neck flask equipped with stirring, reflux, drying device and constant pressure dropping funnel, add 5mL tetrahydrofuran to immerse the magnesium bars. Mix 7.4g of bromohexane (0.045mol) with 60mL of tetrahydrofuran and place in a constant pressure dropping funnel. First add about 1 / 10 volume of the above mixed solution into the flask, and heat until the system obviously reacts. After 10 minutes, under the condition of maintaining reflux, continue to slowly add the rest of the above mixed solution dropwise. After the dropwise addition, maintain reflux and react for about 4 hours. After the reaction, the system was cooled to room temperature. 2.28g POCl 3 (0.015mol) mixed with 20mL tetrahydrofuran, placed in a constant pressure dropping funnel and slowly added dropwise to the above reaction system, and continued to stir for about 1 hour after the dropwise addition was completed. Then add 50 mL of H with a molar conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com