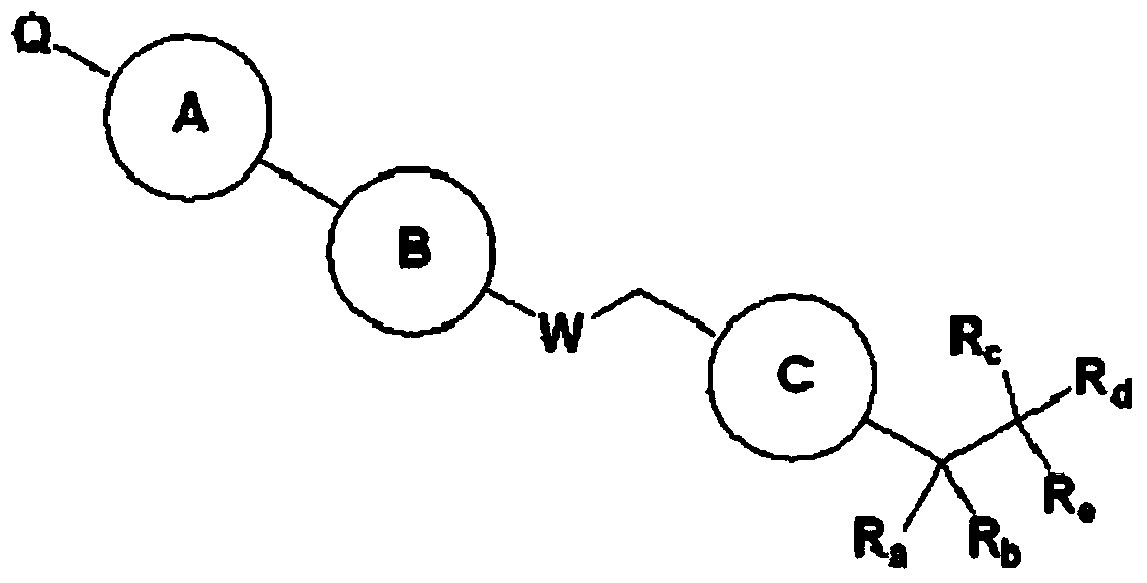
Piperidine derivatives for Gpr119 agonist
A derivative, piperidine technology, applied in the field of piperidine derivatives used as GPR119 agonists, can solve the problems of reduced response to treatment, weight gain, hypoglycemia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0722] 1-(2-fluoro-2-methylpropyl)-4-((4'-(methylsulfonyl)biphenyl-4-yloxy)methyl)piperidine
[0723]
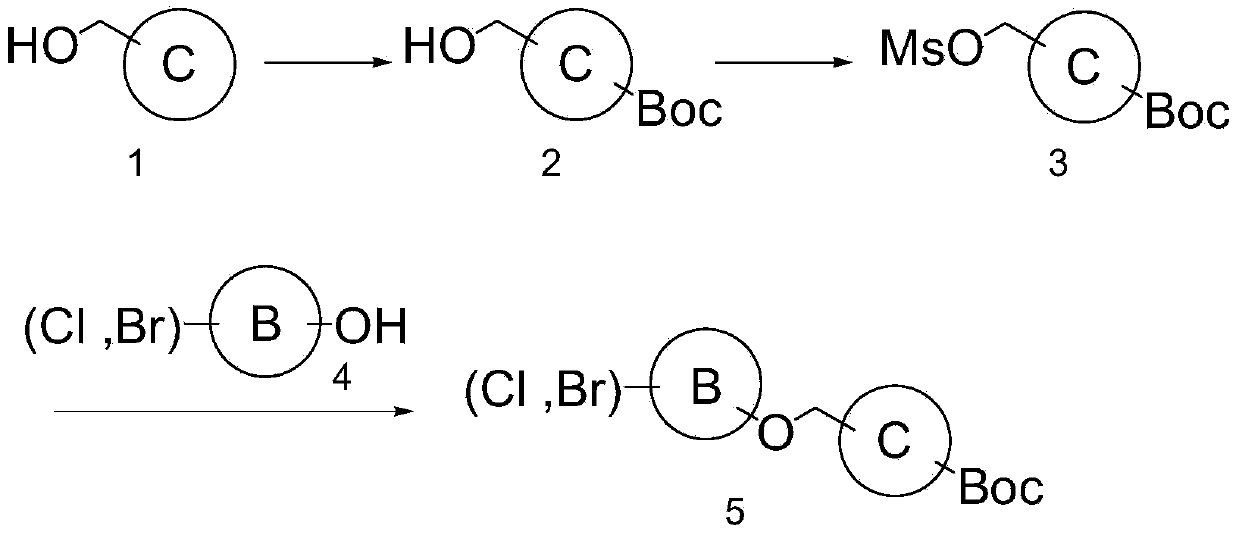
[0724] Step 1. tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate: 4-piperidinemethanol (10.00 g, 86.83 mmol) was dissolved in CH 2 Cl 2 200 mL, and cooled in an ice bath. Di-tert-butyl dicarbonate was added thereto, followed by slowly raising the temperature to room temperature, and stirring for 3 hours. The resulting reaction mixture was sequentially washed with water, saturated NH 4 Cl aqueous solution and saturated saline solution were washed. The washed reaction mixture was washed with MgSO 4 Dry and filter. The solid matter was removed, and then the organic solvent was removed from the filtrate under reduced pressure to give the title compound (18.35 g, 98%) as a white solid.
[0725] Step 2. tert-butyl 4-((methylsulfonyloxy)methyl)piperidine-1-carboxylate: tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate (18.35g, 85.24mmol ) in CH 2 Cl 2 Dissolve in ...
Embodiment 2
[0735]
[0736] Step 1. tert-butyl 4-((5-bromopyridin-2-yloxy)methyl)piperidine-1-carboxylate: Dissolve N-Boc-4-piperidinemethanol (500 mg, 2.32 mmol) in DMF 10 mL . 2,5-Bromopyridine (600 mg, 2.55 mmol) and 95% NaH (83 mg, 3.48 mmol) were slowly added thereto at 0°C, followed by raising the temperature and stirring at room temperature for 3 hours. After completion of the reaction, the reaction mixture was extracted with EtOAc. The resulting organic layer was washed with saturated NH 4 Cl aqueous solution and saturated saline solution were washed three times. The resulting organic layer was washed with Na 2 SO 4 Dry and filter. The filtrate was concentrated under reduced pressure. The resulting concentrate was purified by silica gel column chromatography (0-20% EtOAc / Hexane) to give the title compound (67 mg, 78%) as a white solid.
[0737] Step 2. tert-butyl 4-((5-(4-(methylsulfonyl)phenyl)pyridin-2-yloxy)methyl)piperidine-1-carboxylate: 4-((5-bromopyridine -2-ylox...
Embodiment 3
[0744]
[0745] Step 1. tert-butyl 4-((6-chloropyridin-3-yloxy)methyl)piperidine-1-carboxylate:
[0746] N-Boc-4-piperidinemethanol (0.50g, 2.32mmol) was dissolved in CH 2 Cl 2 5mL, and then slowly dropwise added Et at 0°C 3 N (0.48 mL, 3.48 mmol) and MsCl (0.32 g, 2.79 mmol). The mixture was stirred for 30 minutes, then the temperature was raised and stirred at room temperature for 12 hours. After the reaction was completed, the reaction mixture was washed three times with excess water. The resulting organic layer was washed with Na 2 SO 4 Dry and filter. The filtrate was concentrated under reduced pressure to give the title compound (0.68 g, 100%) as a white solid. The product was dissolved in DMF 10 mL. Slowly add K to it 2 CO 3 (1.13g, 3.48mmol) and 2-chloro-5-hydroxypyridine (0.3g, 2.32mmol). The temperature was raised, and then the mixture was stirred with heating at 100°C for 3 hours. After the reaction was completed, the reaction mixture was washed three...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com