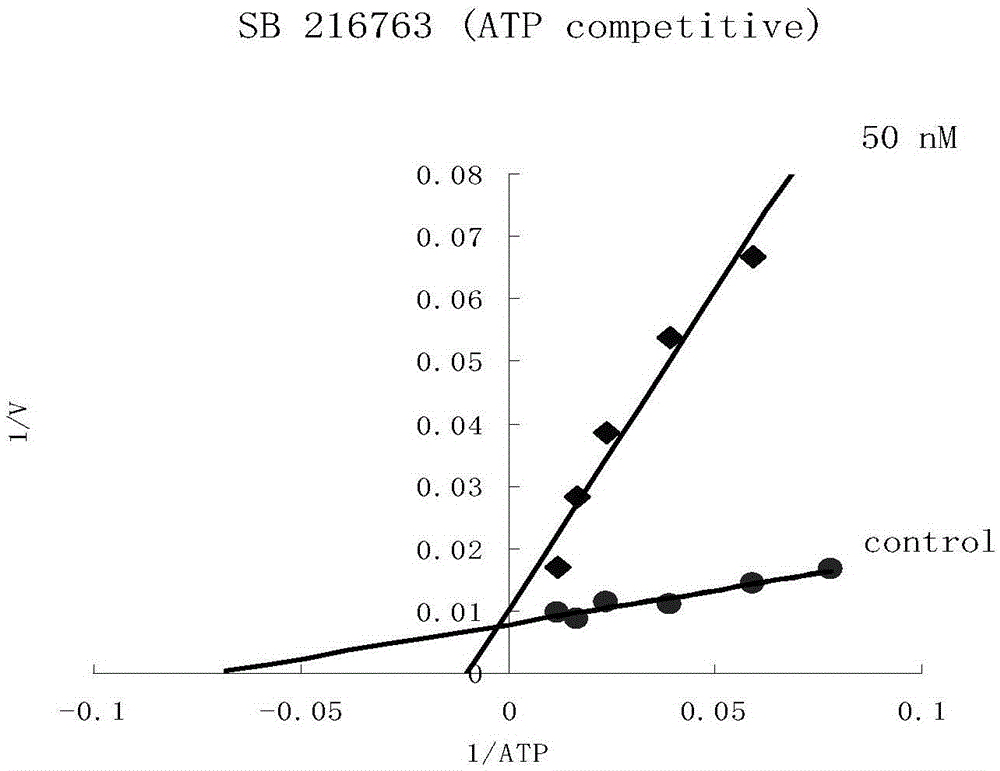
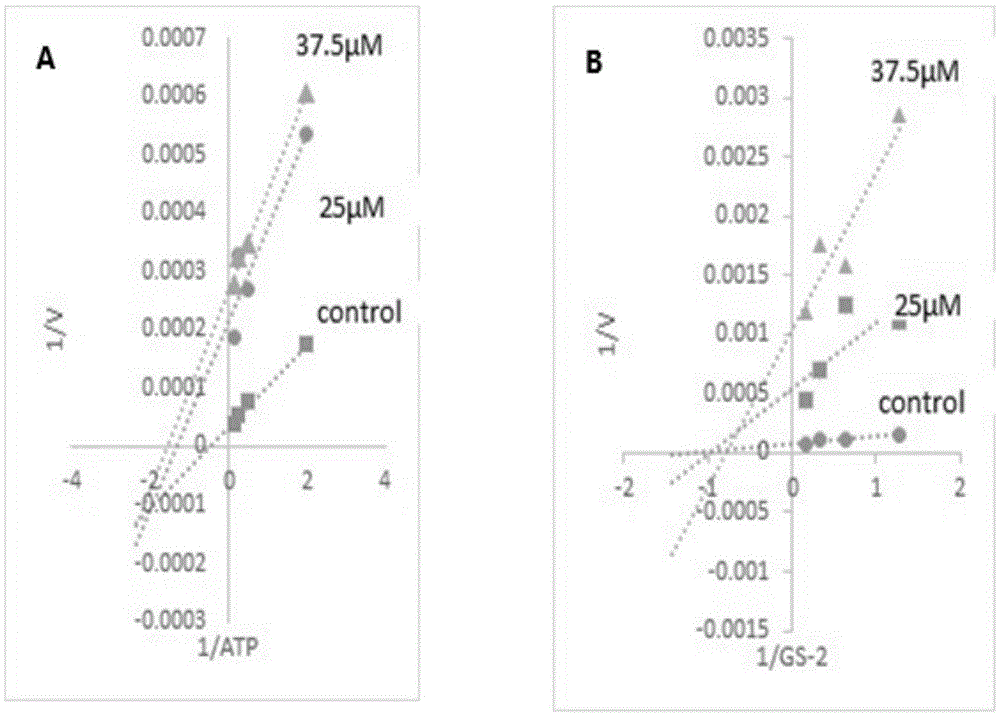
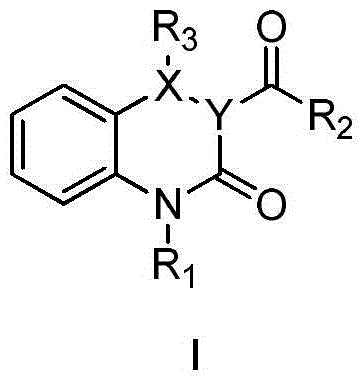
Gsk-3β inhibitor or salt thereof and pharmaceutical use thereof
A -3-oxo-4-, pharmaceutical technology, used in the prevention or treatment of GSK-3β-related diseases, the field of compounds that inhibit glycogen synthase kinase-3β (GSK-3β), and can solve multiple side effects And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of N'-lauroyl-3-oxo-4-benzyl-3,4-dihydro-2H-benzo[1,4]thiazine-2-carbohydrazide (A-11)
[0033]
[0034] (a) Preparation of (2H)1,4-benzothiazin-3(4H)-one (SQa)
[0035] Dissolve 3.0g of o-aminothiophenol (24mmol) in 40ml of anhydrous dichloromethane (DCM), slowly dropwise add chloroacetyl chloride, immediately produce a white precipitate, TLC detection, after 4h o-aminothiophenol disappears, stop the reaction , the solvent was distilled off under reduced pressure, the residue was dissolved in water, extracted with ethyl acetate (100ml×3), the organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, petroleum ether / ethyl acetate (5:1) silica gel Separation by column chromatography yielded 1.06 g of light yellow crystals with a yield of 30%; m.p.182.6~183.8°C. 1 H-NMR (400MHz, DMSO-d 6 )δ10.57(s,1H),7.32(dd,J=8.0,1.4Hz,1H),7.23-7.13(m,1H),6.97(dtd,J=7.8,3.7,1.3Hz,2H),3.46 (s,2H);ESI-MS(m / z)166.0[M+H] + ...
Embodiment 2
[0044] Example 2: Preparation of N'-myristoyl-3-oxo-4-ethyl-3,4-dihydro-2H-benzo[1,4]thiazine-2-carbohydrazide (A-9)
[0045]
[0046] (a) Preparation of 4-ethyl-(2H)1,4-benzothiazin-3(4H)-one (SQb-2)
[0047] The preparation method is the same as that of SQb-1, and the product is a light yellow oil at room temperature, with a yield of 68%. 1 H-NMR (400MHz, CDCl 3 -d 3)δ7.36(dd, J=7.7,1.5Hz,1H),7.26-7.22(m,1H),7.14(dd,J=8.3,1.2Hz,1H),7.02(td,J=7.5,1.2Hz ,1H),4.05(q,J=7.1Hz,2H),3.38(s,2H),1.29(t,J=7.1Hz,3H);ESI-MS(m / z)194.1[M+H] + .
[0048] (b) Preparation of 2-methoxyformyl-4-ethyl-(2H)1,4-benzothiazin-3(4H)-one (SQc-2)
[0049] The preparation method is the same as that of SQc-1, and the product is a colorless oil at room temperature with a yield of 55%. 1 H-NMR (400MHz, CDCl 3 -d 3 )δ7.37(dd, J=7.7,1.5Hz,1H),7.31-7.27(m,1H),7.16(dd,J=8.3,1.2Hz,1H),7.04(td,J=7.6,1.2Hz ,1H),4.26(s,1H),4.22-3.94(m,2H),3.68(s,3H),1.32(t,J=7.1Hz,3H);ESI-MS(m / z)252.1[M +H] + .
[0...
Embodiment 3
[0054] Example 3: Preparation of N'-n-nonanoyl-3-oxo-4-ethyl-3,4-dihydro-2H-benzo[1,4]thiazine-2-carbohydrazide (A-6)
[0055]
[0056] The preparation method is the same as A-9, except that n-nonanoic acid is used instead of n-tetradecanoic acid; petroleum ether / ethyl acetate (2:1) silica gel column chromatography to obtain a white solid powder with a yield of 71.2%. m.p.176.1~178.1℃. 1 H-NMR (400MHz, DMSO-d 6 )δ10.18(d,J=2.1Hz,1H),9.95(d,J=2.1Hz,1H),7.42-7.36(m,1H),7.35-7.27(m,2H),7.06(ddd,J =8.2,5.3,3.2Hz,1H),4.35(s,1H),4.02(dp,J=43.7,7.1Hz,2H),2.06(t,J=7.3Hz,2H),1.46(q,J= 6.8,6.4Hz,2H),1.23(s,10H),1.16(t,J=7.0Hz,3H),0.85(t,J=6.7Hz,3H);ESI-MS(m / z)392.2[M +H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com