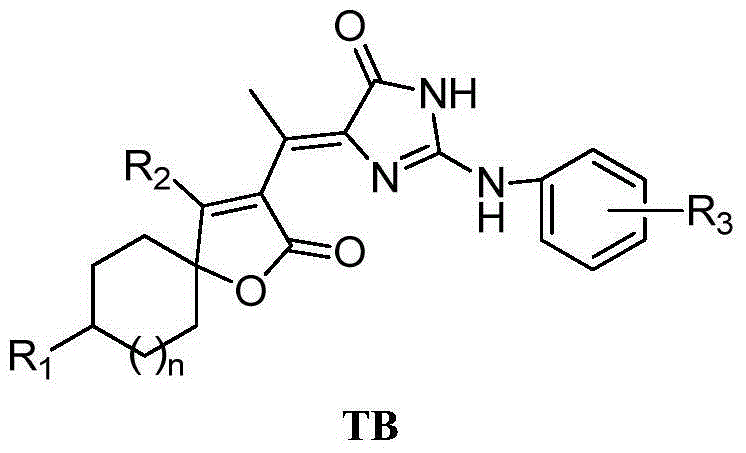
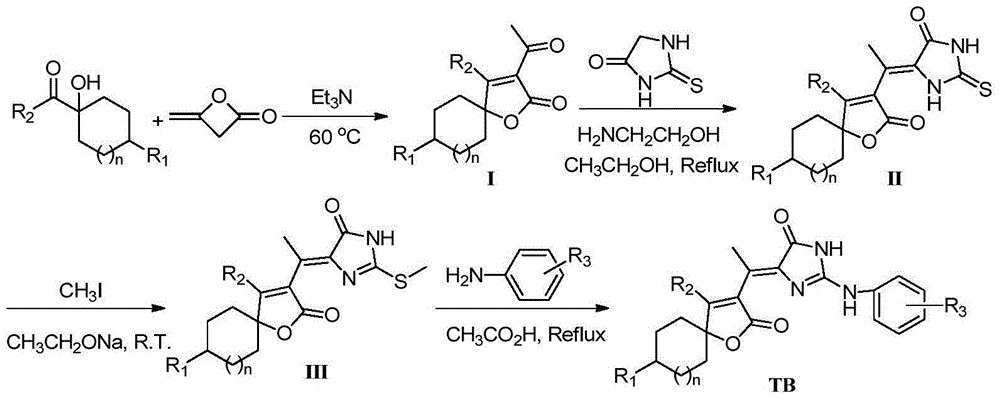
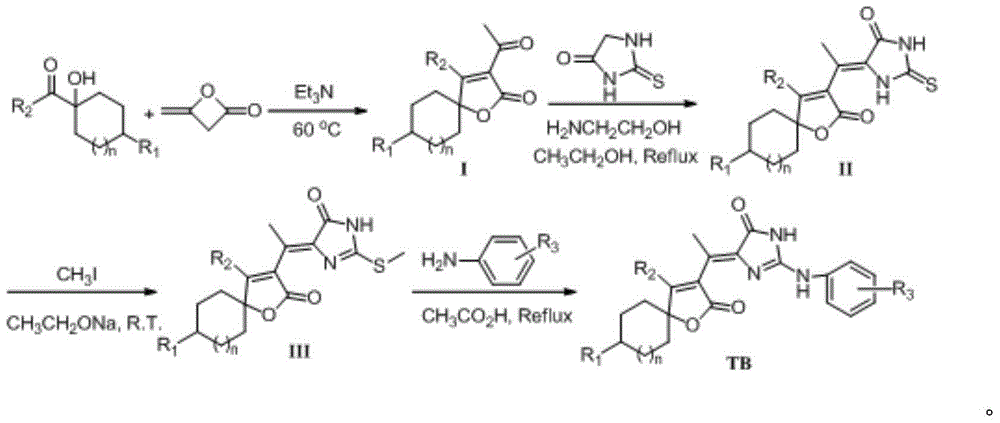
5-(butane lactone-3-ethylidene)-2-amino imidazolinone compounds, preparation method and application thereof
A kind of technology of amino imidazolidinone and butenolide, applied in the field of agricultural chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the synthesis of compound TB-1
[0058]The structural formula of compound TB-1 is as follows:
[0059]
[0060] Among them, n=1, R 1 = H, R 2 =CH 3 , R 3 =H.
[0061] Concrete preparation steps are as follows:
[0062] 1) Synthesis of Compound Ia (n=1, R 1 = H, R 2 =CH 3 ):
[0063] Take 7.1g (50mmol) of 1-hydroxycyclohexyl methyl ketone and 1mL of triethylamine into a 100mL three-necked flask, heat the oil bath to 60°C, add 8.4g (100mmol) of diketene dropwise, and heat to After 12 hours of reaction at 70°C, the reaction was stopped, and the excess liquid was distilled off under reduced pressure. The residue was separated by column chromatography (200-300 mesh silica gel, petroleum ether / ethyl acetate as eluent, 5:1 gradient elution), Obtained 13.34g colorless crystal Ia, yield 51%;
[0064] After testing, Compound Ia: m.p.98~99℃.
[0065] 1 H NMR (300MHz, CDCl 3 )δ:2.57(s,3H),2.33(s,3H),1.86-1.67(m,7H),1.53-1.50(m,2H),1.30-1.23(m,1H). ...
Embodiment 2
[0078] The synthesis of embodiment 2 compound TB-10
[0079] The structural formula of compound TB-10 is as follows:
[0080]
[0081] Among them, n=1, R 1 = H, R 2 =C 6 h 5 , R 3 =H.
[0082] Compound TB-10 was prepared according to the method of Example 1, the difference being: the 1-hydroxycyclohexyl methyl ketone in step 1) was replaced by 1-hydroxycyclohexyl phenyl ketone to obtain compound Ib; correspondingly obtain IIb, IIIb, TB-10.
[0083] After testing, compound Ib was a colorless crystal with a yield of 99%, m.p.137-138°C. 1 H NMR (300MHz, CDCl 3 )δ:7.50-7.45(m,3H),7.20-7.16(m,2H),2.38(s,3H),1.84-1.61(m,9H),1.14-1.13(m,1H).
[0084] Compound IIb: white solid, yield 60%, m.p.284~286℃. 1 H NMR (300MHz, CDCl 3 )δ:12.20(s,1H),11.95(s,1H),7.50-7.43(m,3H),7.25-7.20(m,2H),1.82-1.46(m,12H),1.15-1.06(m, 1H); HR-ESI-MS m / z: C 20 h 21 N 2 o 3 S[M+H] + ,Cacld.369.1267,found369.1258.
[0085] Compound IIIb: yellow crystal, yield 90%, m.p.252~254℃. 1 H NMR (...
Embodiment 3
[0087] The synthesis of embodiment 3 compound TB-19
[0088] The structural formula of compound TB-19 is as follows:
[0089]
[0090] Among them, n=1, R 1 =CH 3 , R 2 =CH 3 , R 3 =H.
[0091] Compound TB-19 was prepared according to the method of Example 1, the difference being that 1-hydroxycyclohexyl methyl ketone was replaced by 1-hydroxyl-4-methylcyclohexyl methyl ketone in step 1) to obtain compound Ic; correspondingly IIc, IIIc, TB-19 were obtained.
[0092] After detection, compound Ic: colorless crystal, yield 61%, m.p.36~37℃. 1 H NMR (300MHz, CDCl 3 )δ:2.56(s,3H),2.41(s,1.5H),2.36(s,1.5H),2.12-1.33(m,9H),1.06(d,J=7.1Hz,1.5H),0.98( d,J=7.1Hz,1.5H).
[0093] Compound IIc: white solid, yield 54%, m.p.273~276℃. 1 H NMR (300MHz, CDCl 3 )δ:12.20-12.12(m,1H),11.96-11.71(m,1H),2.22-1.23(m,15H),1.05-0.92(m,3H); HR-ESI-MS m / z:C 16 h 21 N 2 o3 S[M+H] + ,Cacld.321.1267,found321.1259.
[0094] Compound IIIc: yellow crystal, yield 86%, m.p.151~152℃. 1 H NMR (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com