Pharmaceutical composition for treating gynecological chloasma
A composition and technology of chloasma, applied in the field of medicine, can solve problems affecting the quality of life of patients and achieve remarkable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0014] Embodiment Decoction of the present invention
[0015] Weigh 40 grams of creeping chrysanthemum, 35 grams of cat footprints, 25 grams of long-tube false jasmine, 40 grams of pearl lotus, 30 grams of sticky flower, 20 grams of ephedra, 30 grams of deer ear feathers, 30 grams of lotus leaves, and 20 grams of flowers, 25 grams of madder, 25 grams of Wangjiangnan, 20 grams of Digupi, 30 grams of spring, 25 grams of gardenia, 15 grams of Milletah, 15 grams of scissors, 15 grams of mulberry, 25 grams of pearl wind, 25 grams of pig hairy vegetables, 25 grams of astragalus, 20 grams of coix seed, 15 grams of psoralen, 10 grams of pomegranate, 25 grams of gallinaceous gold, 15 grams of desmodium and 20 grams of licorice, crushed, add 5000 ml of water, and put Heat and reflux in a flask with a condenser for 3 hours, then filter to obtain filtrate 1 and filter residue 1; 2) add 3000 milliliters of water to filter residue 1, heat in a flask with a condenser for 3 hours, filter to o...
experiment example
[0020] normal information
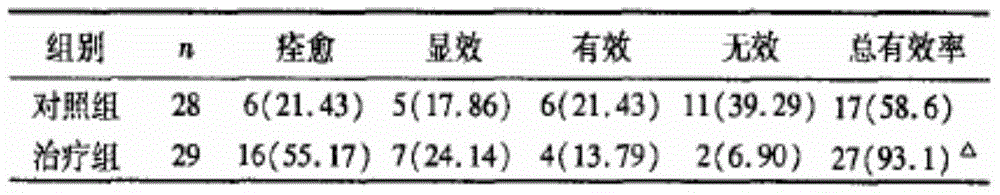
[0021] A total of 57 patients with gynecological chloasma who were admitted to our hospital from January 2013 to May 2014 were selected, aged 29-55 years, all of them were female, and the course of disease ranged from 3 months to 1 year. Divided into two groups, namely the treatment group of 29 cases and the control group of 28 cases. There was no significant difference between the two groups in terms of age, symptoms, course of disease, and severity of the disease (P>0.05), and a controlled trial could be conducted. Diagnosis and syndrome differentiation criteria refer to "Diagnosis and Curative Effect Criteria of TCM Diseases and Syndromes" (State Administration of Traditional Chinese Medicine. TCM Diseases and Syndrome Diagnosis and Curative Criteria. Nanjing: Nanjing University Press, 1994; 156.).
[0022] The control group was treated with Sibai Freckle Ointment (manufacturer: Zhejiang Tianfeng Pharmaceutical Factory, Jinling Pharmaceutical C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com