Diclazuril liposome and preparation method thereof
A technology of diclazuril and zuril fat, which is applied in the direction of liposome delivery, freeze-drying delivery, anti-infective drugs, etc., can solve the problems of insignificant effect, difficult to mix, low solubility, etc., and reduce the amount of clinical use , good stability, guarantee the effect of safe conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
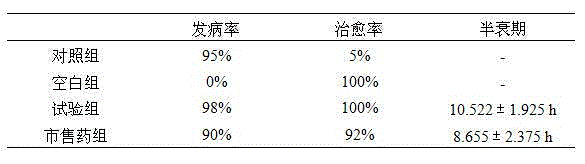
[0022] A liposome of diclazuril, made of the following raw materials in parts by weight: 3-4 parts of diclazuril, 10-20 parts of α-pyrrolidone, 30-50 parts of soybean lecithin, 1-10 parts of glycerin formal 10-20 servings of cholesterol.
[0023] Specific steps are as follows:
[0024] 1) Weigh 3-4 parts of diclazuril raw powder, dissolve it in the mixture of propylene glycol and ethanol, then add PBS buffer solution to fully dissolve it, and make a water phase with a concentration of 3mg / mL for later use ;
[0025] 2) Weigh α-pyrrolidone, soybean lecithin, glycerol formal, and cholesterol in proportion, and use an ultrasonic breaker at normal temperature for 2-5s with an interval of 2-5s to fully dissolve them to obtain the oil phase;
[0026] 3) Evaporate the oil phase under reduced pressure to form a blank phospholipid film, wash and hydrate the blank phospholipid film with ammonium sulfate solution to obtain blank liposomes, filter through a filter membrane, and set asid...
Embodiment 2
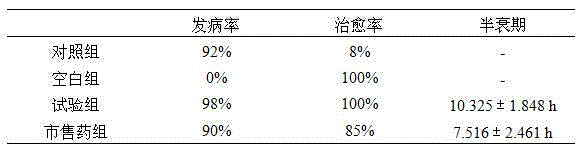
[0035] A diclazuril liposome is prepared from the following raw materials in parts by weight: 30 g of diclazuril, 100 g of α-pyrrolidone, 300 g of soybean lecithin, 10 g of glycerin formal, and 100 g of cholesterol.
[0036] Specific steps are as follows:
[0037] 1) Weigh 30g of diclazuril raw powder, dissolve it in a mixture of propylene glycol and ethanol, the volume ratio of propylene glycol and ethanol is 2:1, and then add PBS buffer solution with a pH value of 6.8-7.2 to make it Fully dissolve to make an aqueous phase with a concentration of 3mg / mL for later use;
[0038] 2) Weigh α-pyrrolidone, soybean lecithin, glycerol formal, and cholesterol in proportion, and act with an ultrasonic crusher at normal temperature for a short time, the action time is 2s, and the interval time is 2s, so that they are fully dissolved and the oil phase is obtained;
[0039] 3) Evaporate the oil phase under reduced pressure to form a blank phospholipid film, wash the blank phospholipid fi...
Embodiment 3
[0043] A diclazuril liposome is prepared from the following raw materials in parts by weight: 32 g of diclazuril, 120 g of α-pyrrolidone, 340 g of soybean lecithin, 30 g of glycerin formal, and 120 g of cholesterol.
[0044] Specific steps are as follows:
[0045] 1) Weigh 32g of diclazuril raw powder, dissolve it in a mixture of propylene glycol and ethanol, the volume ratio of propylene glycol and ethanol is 1:1, and then add PBS buffer solution with a pH value of 6.8-7.2 to make it Fully dissolve to make an aqueous phase with a concentration of 3mg / mL for later use;
[0046] 2) Weigh α-pyrrolidone, soybean lecithin, glycerol formal, and cholesterol in proportion, and act with an ultrasonic breaker at normal temperature for a short time, the action time is 3s, and the interval time is 3s, so that they are fully dissolved to obtain the oil phase;
[0047] 3) Evaporate the oil phase under reduced pressure to make a blank phospholipid film, wash the blank phospholipid film wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com