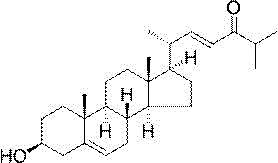
Application of (22trans)-3β-hydroxy-cholesta-5,22-dien-24-one in neuroprotective drugs
A neuroprotective and cholesteric technology, applied in drug combinations, steroids, nervous system diseases, etc., can solve unseen problems and achieve the effect of preventing Alzheimer's disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 (22 trans)-3β - Preparation of Hydroxy-cholesta-5,22-dien-24-one
[0022] (1) Frozen Pteris racemosa ( C. racemosa ) (collected from the coast of Zhanjiang, Guangdong) 5 kg (dry weight) was thawed at room temperature, extracted three times by percolation with 30 L 95% ethanol, each percolation for 24 hours, and combined the extracts;
[0023](2) Concentrate the above extract under reduced pressure at a temperature of ≤45°C to recover ethanol to obtain 350 g of crude extract; disperse the crude extract in water to form a suspension, and use petroleum ether (1.5 L), Ethyl acetate (1.5L) and n-butanol (1L) were extracted three times respectively, and the obtained extracts were concentrated under reduced pressure to obtain petroleum ether extract (38g), ethyl acetate extract (160g) and n-butanol extract ( 120g);
[0024] (3) carry out silica gel column chromatography with ethyl acetate extract, with sherwood oil / acetone gradient elution, the separation compon...
Embodiment 2
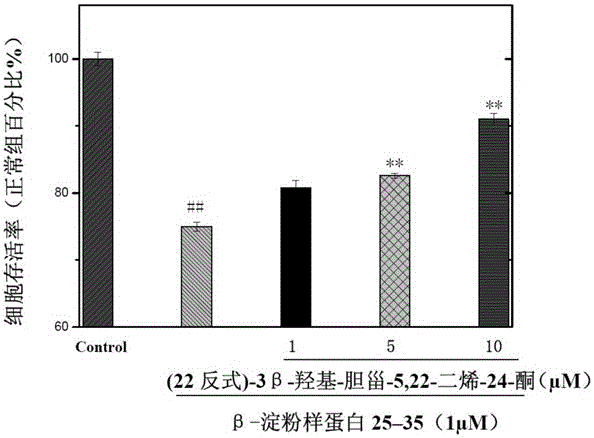
[0025] Example 2 (22 trans)-3β-hydroxyl-cholesta-5,22-dien-24-one on Aβ 25-35 Induced protective effect of SH-SY5Y cell injury
[0026] 1. Experimental samples and experimental methods
[0027] Preparation of test sample solution: The test sample is the pure compound (22trans)-3β-hydroxy-cholesta-5,22-dien-24-one prepared in Example 1 above. Accurately weigh an appropriate amount of sample and prepare a solution with the desired concentration with DMSO for pharmacological activity testing.
[0028] Cell line and cell subculture: The activity test uses the SH-SY5Y cell line (purchased from ATCC (American type culture collection) in the United States). Subculture in DMEM medium containing 10% FBS in an incubator with 5% carbon dioxide at 37°C.
[0029] Cell activity test method (MTT method): the present invention uses the MTT method to test and evaluate the tested sample for Aβ 25-35 Protective effect leading to decreased viability of SH-SY5Y cells. Dehydrogenase in the mit...
Embodiment 3
[0037] Get the compound obtained in Example 1, add commonly used pharmaceutical excipients, and make dosage forms such as tablets, capsules, and oral preparations.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com