Novel morphology controllable CeO2/Cu2O catalyst for CO preferential oxidation reaction through liquid-phase reduction method
A catalyst and reduction technology, which is used in the field of liquid phase reduction method to synthesize a new type of catalyst for CO preferential oxidation reaction CeO2/Cu2O, which can solve the problems of low poisoning ability, high price and limitation, and achieve low price. , wide temperature window, good CO conversion and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 6) Measure CuSO respectively 4 , Na 3 C 6 h 5 o 7 , Na 2 CO 3 , C 6 h 12 o 12 Add 20ml of each solution into a three-necked flask, add 400ml of deionized water, heat to 80°C, and mechanically stir for 40min.
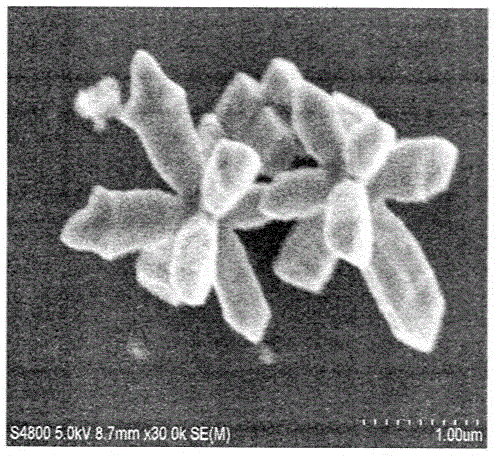
[0040] 7) Wash the obtained precipitate by centrifugation. Wash three times with water first, and then wash three times with absolute ethanol. The precipitate was vacuum-dried at 60 °C for 12 h to obtain star-shaped Cu 2 O carrier.
[0041] 8) Take 1g (6.988mmol) of the prepared star-shaped Cu 2 Add distilled water dropwise to the O carrier until the incipient wetness, and record its water absorption as 0.5ml, which is the minimum amount of water required to wet the carrier.
[0042] 9) Take 6.988mmol Ce(NO 3 ) 3 ·6H 2 O was dissolved in 0.5ml distilled water and ultrasonicated until completely dissolved to obtain Ce(NO 3 ) 3 solution.
[0043] 10) Take 1g of the prepared star-shaped Cu 2 O carrier to Ce(NO 3 ) 3 solution, soaked for 24 hours...
Embodiment 2
[0046] 6) Measure 20ml CuSO 4 Add the solution into a three-necked flask, add 400ml of deionized water to dissolve, add 20ml of CTAB, heat to 80°C, stir mechanically for 10-15min, then add 20ml of Na 3 C 6 h 5 o 7 and 20ml Na 2 CO 3 Mix the solution, after 10min, add 20ml C 6 h 12 o 12 The solution was mechanically stirred for 30 min.
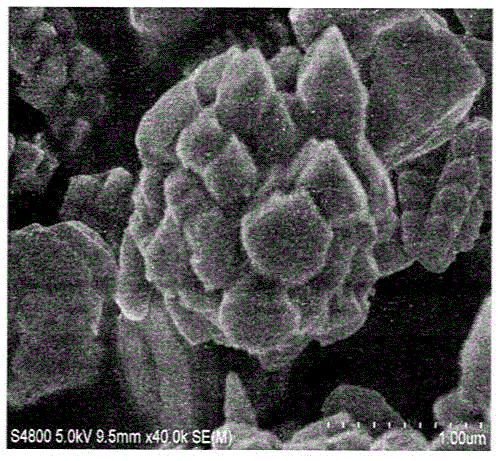
[0047] 7) Wash the obtained precipitate by centrifugation. Wash three times with water first, and then wash three times with absolute ethanol. The precipitate was vacuum-dried at 60 °C for 12 h to obtain hollow spheres Cu 2 O carrier.
[0048] 8) Take 1g (6.988mmol) of the prepared hollow spherical Cu 2 Add distilled water dropwise to the O carrier until the incipient wetness, and record its water absorption as 0.5ml, which is the minimum amount of water required to wet the carrier.
[0049] 9) Take 6.988mmol Ce(NO 3 ) 3 ·6H 2 Ce(NO 3 ) 3 solution.
[0050] 10) Take 1g of the prepared hollow spherical Cu 2 The O carrier is d...
Embodiment 3
[0053] 6) Measure CuSO respectively 4 , Na 3 C 6 h 5 o 7 , Na 2 CO 3 , C 6 h 12 o 12 Add 20ml of each solution into a three-necked flask, add 400ml of deionized water, add 20ml of 2.0mM CTAB, heat to 80°C, and mechanically stir for 40min.
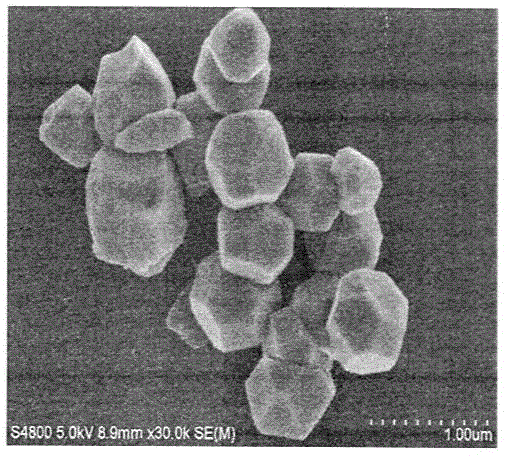
[0054] 7) After the obtained solution is fully precipitated, it is centrifuged and washed. Wash three times with water first, and then wash three times with absolute ethanol. The precipitate was vacuum-dried at 60 °C for 12 h to obtain polyhedral Cu 2 O carrier.
[0055] 8) Take 1g (6.988mmol) of the prepared polyhedral Cu 2 Add distilled water dropwise to the O carrier until the incipient wetness, and record its water absorption as 0.5ml, which is the minimum amount of water required to wet the carrier.
[0056] 9) Take 6.988mmol Ce(NO 3 ) 3 ·6H 2 Ce(NO 3 ) 3 solution.
[0057] 10) Take 1g of the prepared polyhedral Cu 2 The O carrier is dissolved in the Ce(NO 3 ) 3 solution, soaked for 24 hours and then vacuum-dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com