Novel diazo benzothiapyrone photosensitive protecting groups and synthesis method thereof
A technology of benzothiopyrone and diazobenzene, which is applied in the field of preparation of new compounds, can solve the problems of increased difficulty in experimental operation, inconvenient experiment, and reduced reaction yield, and achieves efficient and rapid photodissociation reactions with good relative The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] A preparation of 1,1-dioxo-2-diazo-3-phenyl-4H-benzothiopyrone, the specific steps are as follows:
[0060] ⑴2-Methyl-4H-benzothiopyrone
[0061] 12.5g H 3 PO 4 , 18gP 2 o 5 Stir at 70°C, after dissolving, add the mixture of 1.5mL thiophenol and 1.5mL ethyl acetoacetate dropwise (15min) with a constant pressure dropping funnel, react for 3 hours, pour into ice water, extract with ethyl acetate, The organic layer was washed with 10% NaOH aqueous solution (20mL×3), adjusted to pH=9, after washing with water, the organic layer was washed with anhydrous MgSO 4 After drying, the obtained product was concentrated and crystallized with ether to obtain 1.6 g of yellow crystals, with a yield of 77.4%.
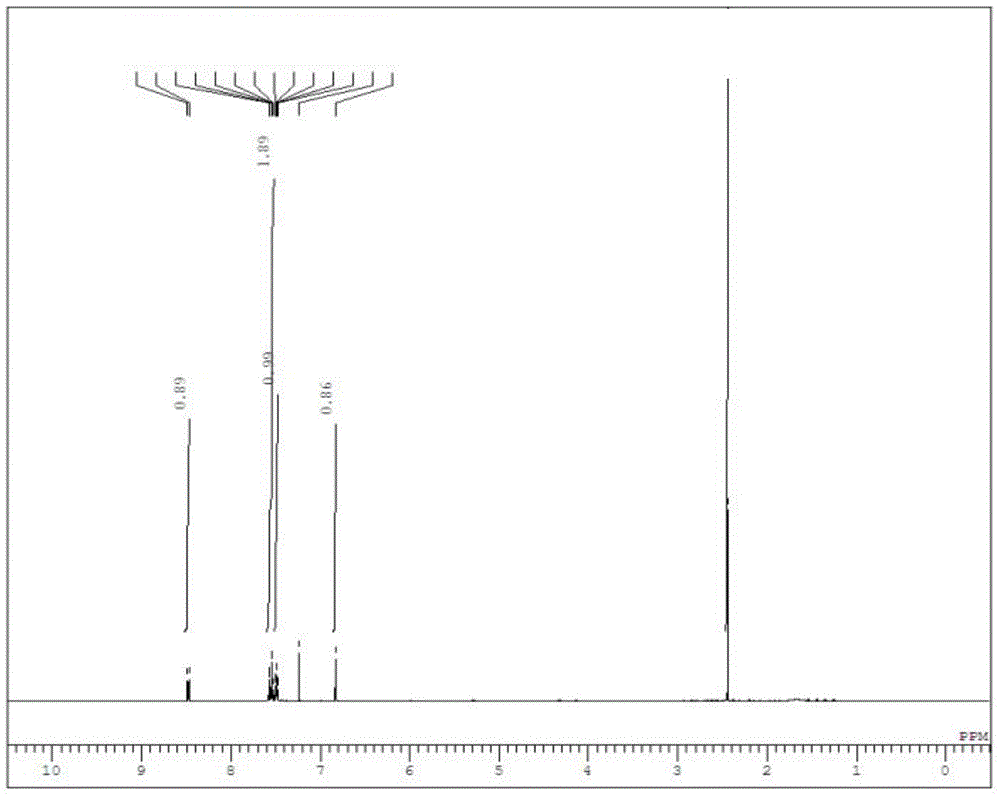
[0062] 1 H-NMR (CDCl 3 / TMS) δ: 8.48(1H, J=7.9Hz, d), 7.56-7.52(2H, m), 7.50(1H, J=7.6, 7.9Hz, dd), 6.82(1H, s), 2.44(3H ,s).
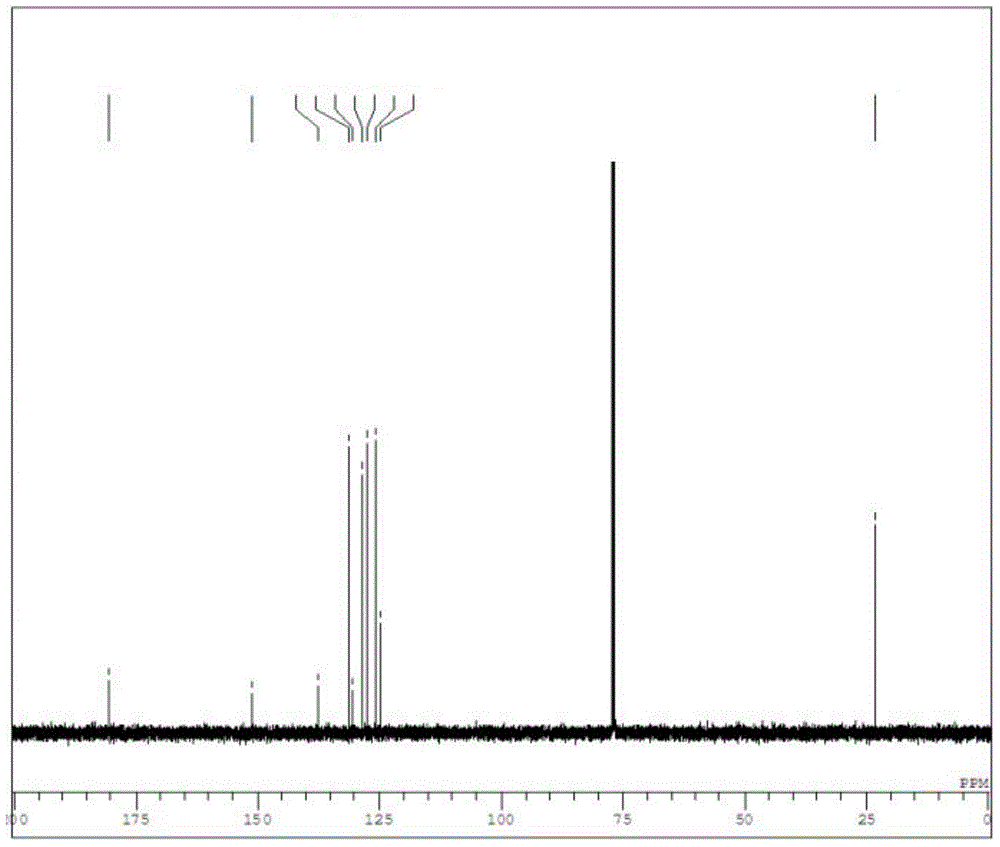
[0063] 13 C-NMR (CDCl 3 )δ: 180.63, 151.32, 137.64, 131.35, 130.70, 128.54, 127.48, 125.98, 124.89, 23.31.
[0064] ⑵3-iodo-2-methyl-4H-benz...
Embodiment 2
[0089] 1,1-dioxo-2-diazo-3-p-methoxyphenyl-4H-benzothiopyrone was synthesized by the same method as in Example 1, and the yield was 84%.
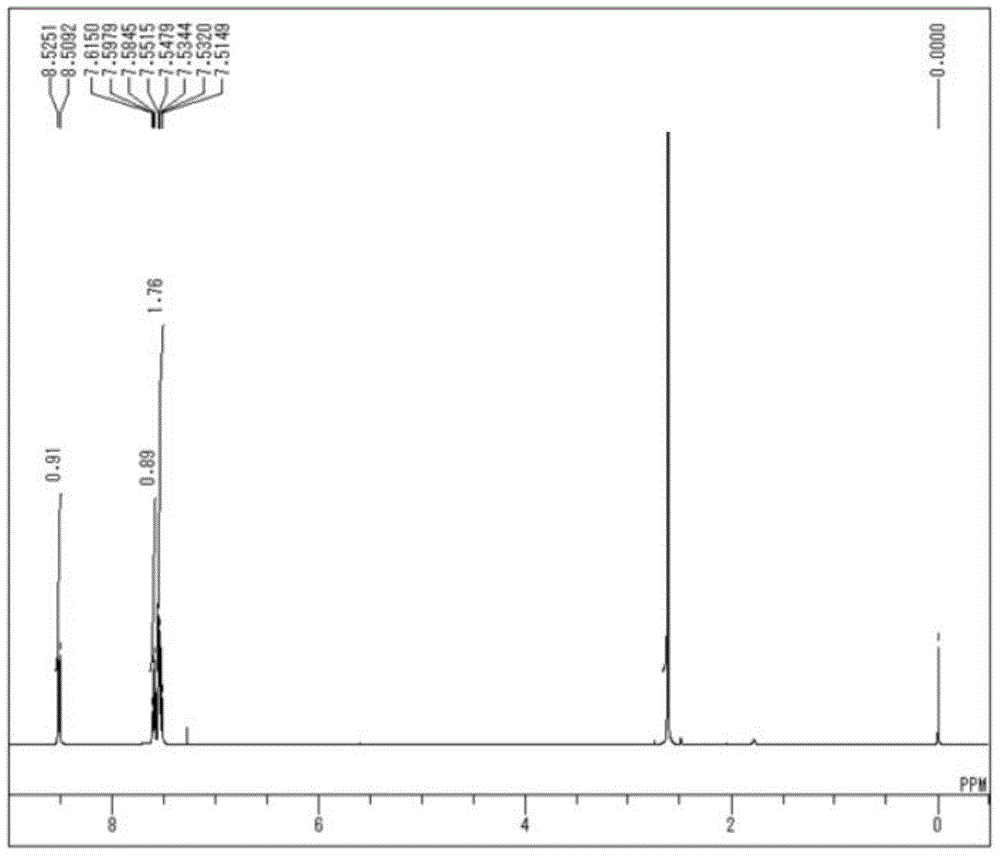
[0090] 1 H-NMR (CDCl3) δ: 8.21 (1H, d, J = 7.9Hz), 8.09 (1H, d, J = 7.9Hz), 7.89 (1H, dd, J = 7.9, 7.9Hz), 7.78 (1H, dd,J=7.9,7.9Hz),7.35(2H,d,J=9.2Hz),7.00(2H,d,J=9.2Hz),5.03(1H,s),3.86(3H,s).
[0091] 13 C-NMR (CDCl 3 )δ: 178.94, 160.63, 147.08, 142.50, 139.96, 134.54, 133.33, 131.18, 129.22, 129.03, 122.99, 122.66, 113.80, 55.32, 47.01.
Embodiment 3
[0093] The synthesis of 1,1-dioxo-2-diazo-3-(3,5-bis(trifluoromethyl))phenyl-4H-benzothiopyrone, the method is the same as in Example 1, and the yield 75%.
[0094] 1 H-NMR (CDCl 3 )δ: 8.25 (1H, d, J = 7.9Hz), 8.13 (1H, d, J = 7.9Hz), 8.02 (1H, s), 7.96 (1H, dd, J = 7.9, 7.9Hz), 7.92 ( 2H,s),7.85(1H,dd,J=7.9,7.9Hz),5.05(1H,s).
[0095] 13 C-NMR (CDCl 3 )δ: 177.79, 149.68, 139.85, 139.81, 135.22, 133.74, 132.58, 132.00, 131.73, 131.46, 130.20, 129.23, 128.56, 124.04, 123.39, 121.87, 46.54.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com