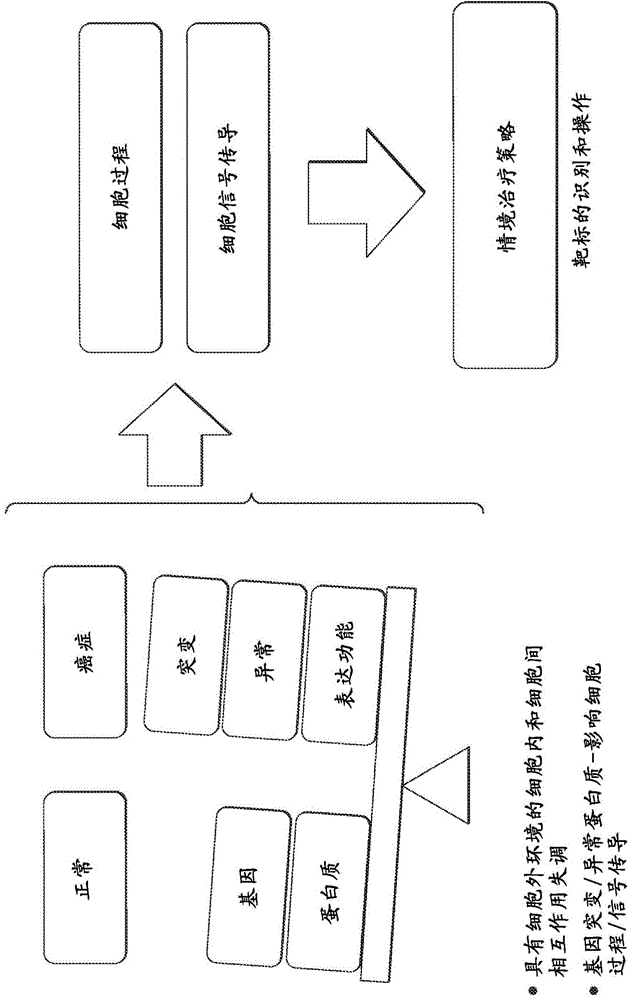
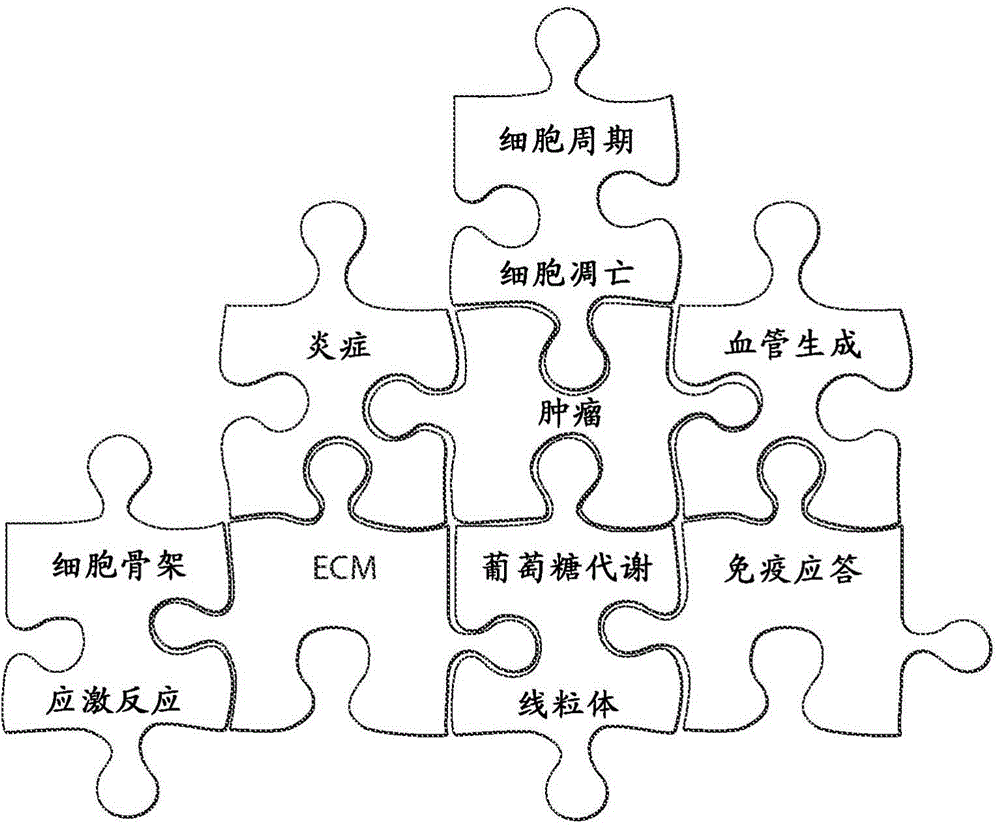
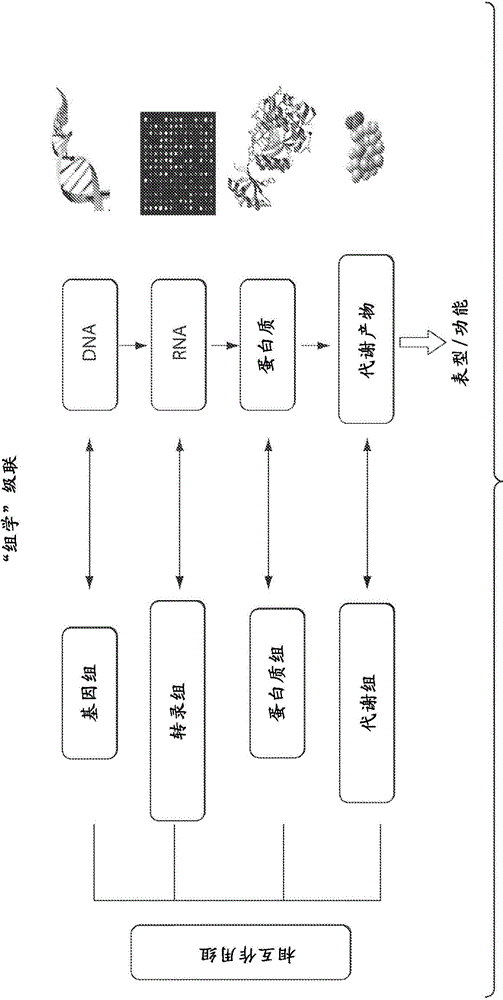
Interrogatory cell-based assays for indentifying drug-induced toxicity markers
A technology of biomarkers and drugs, which can be used in drug combination, microbe measurement/testing, antiviral agents, etc., and can solve complex problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0264] Preparation of secretome samples: 1) In one embodiment, the cells can be cultured in a serum-free medium: the conditioned medium can be concentrated by a lyophilizer, reduced (10 mM dithiothreitol (DTT), 55° C., 1 hour), alkylation (25 mM iodoacetamide, at room temperature, incubation for 30 minutes) and then desalted by acetone precipitation. Equal amounts of protein from concentrated conditioned media can be digested with trypsin (1:25 w / w, 200 mM triethylammonium bicarbonate (TEAB), 37°C, 16 hours).
[0265] In one embodiment, cells can be cultured in serum-containing medium: the volume of the medium can be reduced using 3kMWCO Vivaspin columns (GE Healthcare Life Sciences) and then can be reconstituted with IxPBS (Invitrogen). Serum albumin can be depleted from all samples using AlbuVoid columns (Biotech Support Group, LLC) with buffer exchange modified to optimize conditioned medium application following the manufacturer's instructions.
[0266] iTRAQ 8-plex label...
Embodiment 1
[0595] Example 1: Establishment of a drug-induced cardiotoxicity model using platform technology
[0596] In this example, the platform technology described in detail in International PCT Application No. PCT / US2012 / 027615 was employed to integrate data obtained from custom drug-induced cardiotoxicity models and to identify novel mechanisms driving pathogenesis / drug cardiotoxicity. protein / pathway. The relationship map resulting from this analysis has provided biomarkers of drug-induced cardiotoxicity.
[0597] In a healthy heart, systolic function depends on the balance of fatty acid and carbohydrate oxidation. Chronic dysregulation of uptake, utilization, organelle biosynthesis and secretion in non-adipose tissues (heart and liver) is thought to be central to mitochondrial damage and dysfunction and a key player in drug-induced cardiotoxicity. Applicants describe here a systematic approach combining protein and lipid imprinting with functional endpoint analysis focused spec...
Embodiment 2
[0615] Example 2: Identification of additional cardiotoxicity markers using a drug-induced cardiotoxicity model
[0616] Further data obtained from the same custom cardiotoxicity model were similarly integrated using the platform technology described in Example 1 above. Cardiotoxicity was modeled using five patient cardiomyocyte lines, as explained in the detailed description above. These five cardiomyocyte lines were then subjected to mitochondrial ATP analysis to analyze mitochondrial dysfunction induced by drug treatment or the absence of drug treatment (indicated as + and −) in diabetic state (hyperglycemia) and normoglycemia (normoglycemia). A decrease in mitochondrial ATP was observed in the diabetic state upon drug treatment only in 2 of 5 cardiotoxicity models (see Figure 30 ). The results of these further experiments led to the identification of additional novel proteins / pathways driving the pathology of drug cardiotoxicity, such as Figure 26-34 summarized in.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com