Beta site amyloid protein precursor (APP)-cleaving enzyme (BACE1) inhibitor containing pyridine ring
A technology of amyloid protein and inhibitors, which is applied in the fields of nervous system diseases, organic chemistry, drug combination, etc., and can solve the problems of compound 1, such as strong fat solubility and little medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
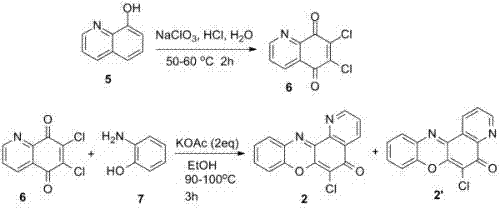
[0021] 6,7-dichloro-5,8-quinoline diquinone (compound 6 ) preparation
[0022] First 1.5 grams of 8-hydroxyquinoline were dissolved in 60 milliliters of concentrated hydrochloric acid, and kept stirring, then slowly added 5.3 grams of sodium chlorate to this concentrated hydrochloric acid solution containing 8-hydroxyquinoline. After the reaction mixture was stirred at 50-60° C. for 2 hours, 200 ml of distilled water was added for dilution. The yellow solid formed was removed by filtration. The filtrate was extracted three times with 50 ml of dichloromethane (3×50 mL), the organic phase was washed once with water, and the dichloromethane was distilled off. The crude product was recrystallized in methanol to give pure bright yellow crystals of 6,7-dichloro-5,8-quinoline diquinone (compound 6 ) 0.95 g. 1 HNMR (Varian HFT-80) (Me 2 SO-d 6 ) δ(ppm) 9.05 (dd, J 2,3 = 4.8 Hz, J 2,4 = 1.6 Hz, 1 H), 8.46 (dd, J 3,4 = 8.0 Hz, J 2,4 = 1.6 Hz, 1 H), 7.88 (dd, J 3,4 = 8.0...
Embodiment 2
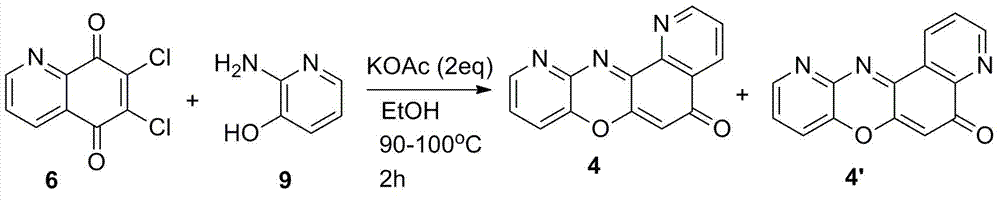
[0024] 6-Chloro-7-oxa-1,12-diaza-benzo(h)anthracen-5-one (compound 2 ) and 6-chloro-7-oxa-4,12-diaza-benzo(h)anthracen-5-one (compound 2’ ) preparation
[0025] To a flask containing 20 ml of absolute ethanol, add 1.15 g of 6,7-dichloro-5,8-quinoline diquinone, 0.54 g of o-hydroxyaniline, and 0.98 g of potassium acetate, respectively, and keep stirring. The reaction mixture was refluxed for 3 hours under continuous stirring. After the reaction, cool down, add ice water, stir, and filter. The obtained dark brown solid was first washed with cold water, washed with cold ethanol, and dried to obtain 0.95 g of 6-chloro-7-oxa-1,12-diaza-benzo(h)anthracene-5 - Keto (compound 2 ) and 6-chloro-7-oxa-4,12-diaza-benzo(h)anthracen-5-one (compound 2’ )mixture. ESI-MS m / z: 282.01 (C 15 h 7 ClN 2 o 2 Calculated value 282.02).
Embodiment 3
[0027]6-Chloro-7-oxa-4,12-diaza-benzo(h)anthracene-5-one (compound 3 ) preparation
[0028] Under constant stirring, 2.27 grams of 2,3-dichloro-1,4-naphthalene diquinone, 1.10 grams of 2-amino-3-hydroxypyridine, and 1.96 grams of acetic acid were added to a flask containing 50 milliliters of absolute ethanol. potassium. The reaction mixture was refluxed for 2 hours under continuous stirring, cooled, added with ice water, stirred, and filtered. The resulting dark brown solid was washed several times with cold water and ethanol, and dried to obtain 2.12 g of 6-chloro-7-oxa-11,12-diaza-benzo(h)anthracene-5-one (compound 3 ). 1 HNMR (Bruker DX400) (CDCl 3 )δ (ppm) 7.61 (m, 3H), 7.85 (m, 3H), 8.4 (m, 1H), 8.75 (m, 1H), 8.91 (m, 2H). ESI-MS m / z: 282.01; Calcd. C 15 h 7 ClN 2 o 2 282.02.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com