Application of pharmaceutical composition in preparation of medicine for treating sepsis
A composition and sepsis technology, applied in the direction of drug combination, active ingredients of hydroxyl compounds, antipyretics, etc., can solve problems such as increased intestinal mucosal permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Replicate mouse CLP sepsis (severe) model
[0033] Male BALB / c mice, weighing 25-30 g, were depilated from the abdomen the day before the experiment. Pentobarbital sodium 40 mg / kg was injected intraperitoneally, fixed after anesthesia, and disinfected with iodine; a 1.5 incision was made along the midline of the abdomen to find the cecum, the mesentery was carefully stripped, and the cecum was ligated at the root of the cecum to avoid ligation of the ileum and cecal mesenteric vessels. The cecum was punctured three times with a 16-gauge needle, and a small amount of intestinal content was squeezed out. Then the cecum was returned to the abdominal cavity, and the abdominal wall incision was sutured layer by layer. After the operation, penicillin eye ointment was applied to prevent infection, and the wound was bandaged with a band-aid. About 20 minutes after the operation was completed, the mice regained their mobility. Before the CLP operation, the mice were ...
Embodiment 2
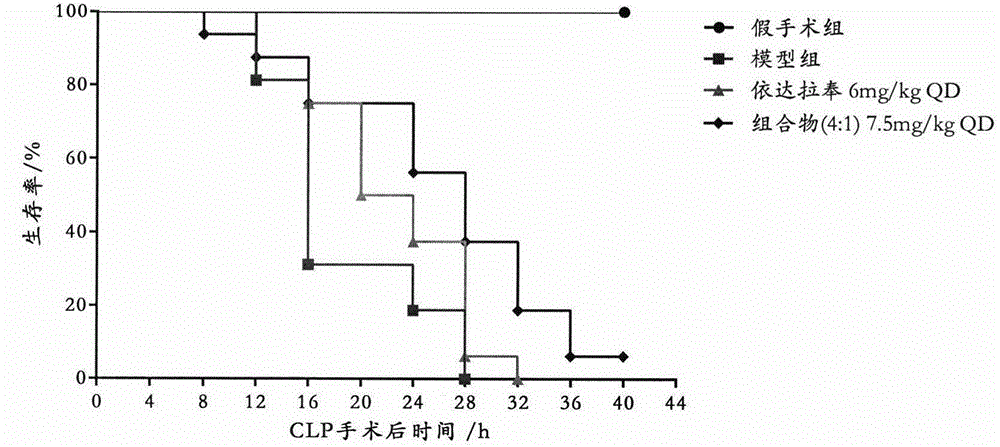
[0034] Embodiment 2: Effect of a single administration of the composition (4:1) on the survival rate of CLP model mice
[0035] BALB / c mice, male, 64, were randomly divided into sham operation group, sepsis model group, Edaravone treatment group (single i.v., 6mg / kg) and composition (4:1) treatment group ( Single i.v., 7.5mg / kg).
[0036] Composition (4:1) means Edaravone 3mg / kg+natural borneol 0.75mg / kg.
[0037] Edaravone injection, specification 5mL: 10mg (contains edaravone 10mg), produced by Nanjing Simcere Dongyuan Pharmaceutical Co., Ltd.; composition (4:1) injection, specification 5mL: 12.5mg (contains Edaravone 10mg and natural borneol 2.5mg, dissolved in 8% 1,2,-propanediol aqueous solution). Both are diluted to the required concentration with normal saline before use. 30 minutes after CLP modeling, the tail vein was injected slowly (<5 minutes).
[0038] The above-mentioned 4 groups of animals were counted every 4 hours after the death of animals, and the result...
Embodiment 3
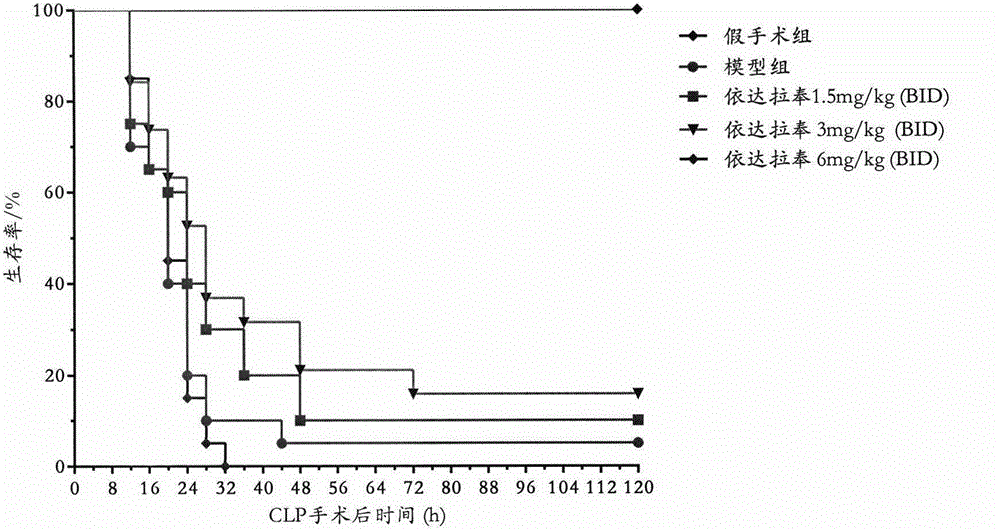
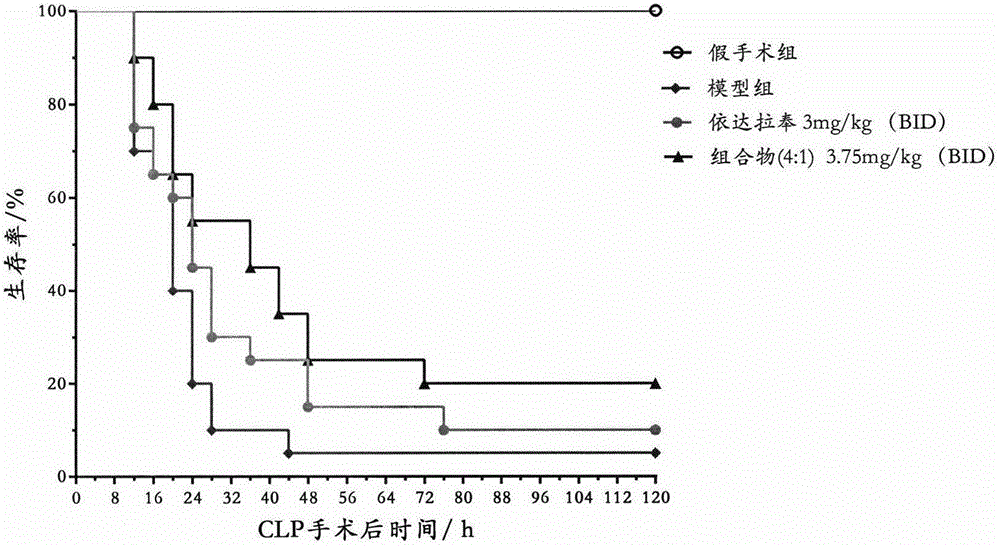
[0041] Embodiment 3: Replicate rat CLP sepsis model
[0042] Male SD rats, weighing 250-280g, were anesthetized by intraperitoneal injection of pentobarbital sodium 40mg / kg, fixed, depilated, disinfected, and covered with sterile drapes. Make a 1.5 cm incision along the midline of the abdomen, find the cecum, carefully peel off the mesentery, and ligate the cecum at the root of the cecum to avoid ligating the ileum and cecal mesenteric vessels. The cecum was punctured twice with a 16-gauge needle, and a small amount of intestinal content was squeezed out. Then the cecum was returned to the abdominal cavity, and the abdominal wall incision was sutured layer by layer, and the operation was completed. Immediately subcutaneously inject 10 mL of preheated normal saline into the animal for anti-shock. Before the CLP operation, the rats were lively and active, with smooth fur, rosy lips and front and rear paws, active eating and drinking, and normal defecation; after the CLP operat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com