Difenoconazole copper complex as well as preparation method and application thereof
A technology of difenoconazole and metroconazole copper, which is applied in the direction of botany equipment and methods, copper organic compounds, applications, etc., can solve the problems of apple yield and quality impact, fruit tree failure, etc., to improve biological activity and reduce Dosage, the effect of expanding the bactericidal spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
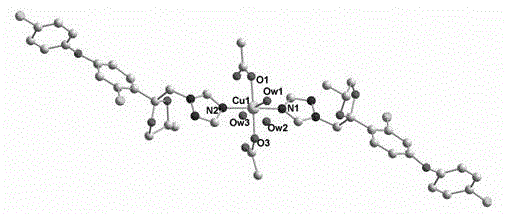
[0027] Example 1 Complex (1) [Cu(C 19 h 17 Cl 2 N 3 o 3 ) 2 (CH 3 COO) 2 (H 2 O)] 2H 2 Synthesis of O
[0028] Dissolve 0.22mmol (0.0894g) of difenoconazole in 10mL of absolute ethanol, dissolve 0.1mmol (0.0100g) of copper acetate in 5mL of distilled water, slowly add the aqueous solution of copper acetate into the ligand solution, and stir at room temperature 1h, filter, collect the filtrate in a beaker, and after standing at room temperature for a week, blue blocky crystals appear. Elemental analysis (C 42 h 46 C l4 N 6 o 13 Cu): Calculated (%): C, 48.13; H, 4.42; N, 8.02; Found (%): C, 48.09; H, 4.41; N, 7.99.
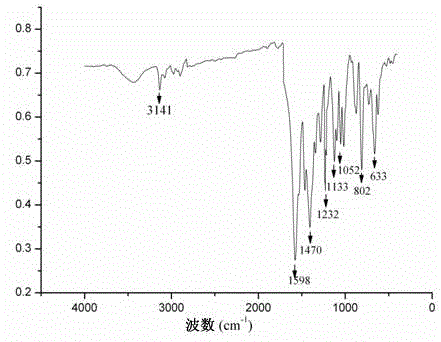
[0029] Such as figure 1 As shown, the main infrared characteristic absorption peak of complex 1 is 3141cm -1 (ν =C–H ), 1598cm -1 (ν C=N ), 1470cm -1 (ν C=C ), 1232cm -1 (ν Ar–O–Ar ), 1133 cm -1 (ν C–N ), 1052cm -1 (ν R–O–R ), the stretching vibration absorption peaks of =C–H, C=N, C–N in the ligand are 3101cm -1 、1591cm -1 、1130 cm ...
Embodiment 2
[0032] Example 2 Complex (2) [Cu(C 19 h 17 Cl 2 N 3 o 3 ) 2 (COOH) 2 ]·H 2 Synthesis of O
[0033] Dissolve 0.11mmol (0.0447g) of difenoconazole in 10mL of absolute ethanol, dissolve 0.1mmol (0.0113g) of copper formate in 5mL of distilled water, slowly add the aqueous solution of copper formate into the ligand solution, and stir at room temperature 1h, filter, collect the filtrate in a beaker, and after standing at room temperature for three weeks, blue blocky crystals appear. Elemental analysis (C 40 h 38 C l4 N 6 o 11 Cu): Calculated (%): C, 48.82; H, 3.89; N, 8.54; Found (%): C, 48.79; H, 3.81; N, 8.49.
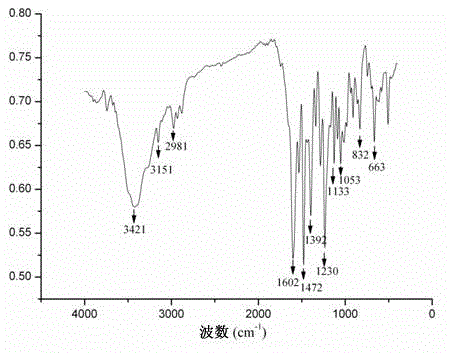
[0034] Such as image 3 As shown, the main infrared characteristic absorption peak of complex (2) is 3151cm -1 (ν =C–H ), 1602cm -1 (ν C=N ), 1472cm -1 (ν C=C ), 1230cm -1 (ν Ar–O–Ar ), 1133 cm -1 (ν C–N ), 1053cm -1 (ν R–O–R ), the stretching vibration absorption peaks of =C–H, C=N, C–N in the ligand are 3101cm -1 、1591cm -1 、1130 cm -1 . ...
Embodiment (3)
[0037] Embodiment (3) Complex (3) Cu(C 19 h 17 Cl 2 N 3 o 3 ) 2 (NO 3 ) 2 Synthesis
[0038] Dissolve 0.11mmol (0.0447g) of difenoconazole in 10mL of absolute ethanol, and dissolve 0.1mmol (0.0121g) of copper nitrate in 5mL of distilled water. Slowly add the aqueous solution in which copper nitrate is dissolved into the ligand solution, and stir at room temperature 1h, filter, collect the filtrate in a beaker, and after standing at room temperature for three weeks, blue blocky crystals appear. Elemental analysis (C 38 h 34 C l4 N 8 o 12 Cu): Calculated (%): C, 45.64; H, 3.43; N, 11.20; Found (%): C, 45.59; H, 3.41; N, 11.19.
[0039] Such as Figure 5 As shown, the main infrared characteristic absorption peak of complex (3) is 3139cm -1 (ν =C–H ), 1605cm -1 (ν C=N ), 1472cm -1 (ν C=C ), 1230cm -1 (ν Ar–O–Ar ), 1133 cm -1 (ν C–N ), 1002cm -1 (ν R–O–R ), the stretching vibration absorption peaks of =C–H, C=N, C–N in the ligand are 3101cm -1 、1591cm -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com