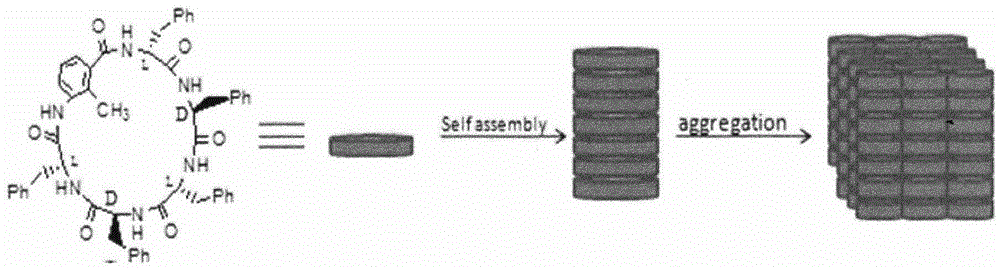
Novel cyclic peptide containing 2-methyl-3-aminobenzoic acid
A technology of aminobenzoic acid and amino acid, applied in the field of nanotubes, can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
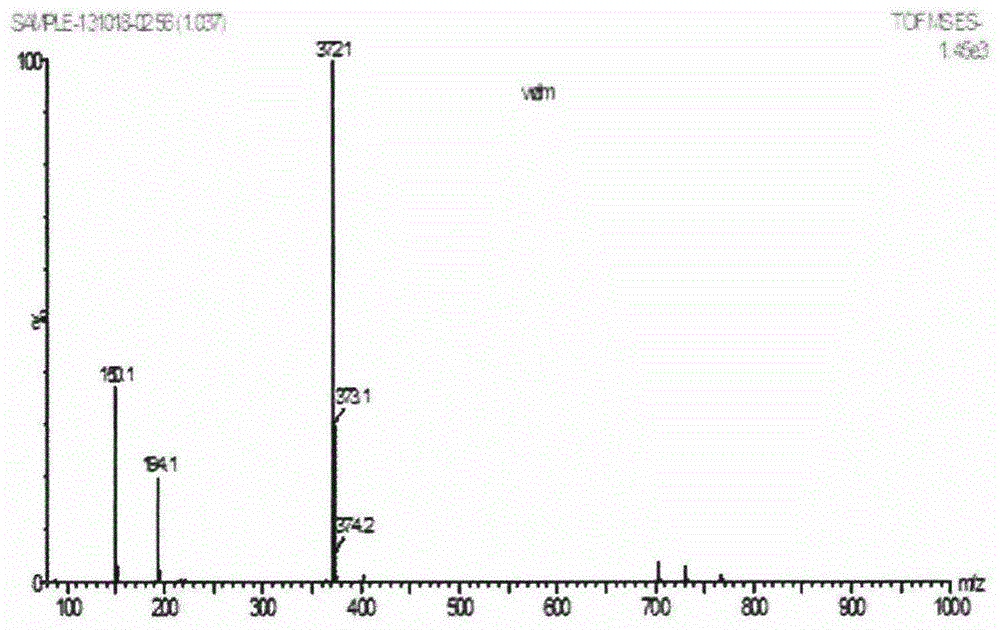
[0022] Example 1 Synthesis of 3-(9-fluorenylmethoxycarbonyl)amino-2-methylbenzoic acid (Fmoc-γ-Mba)
[0023] Dissolve γ-Mba (1mmol, 0.15116g) in distilled water, Na 2 CO 3 (1mmol, 0.106g) was slowly added thereto until all of the γ-Mba was dissolved; 9-fluorenylmethylsuccinimidyl carbonate (Fmoc-OSu, 1.05mmol, 0.3542g) was dissolved in 5ml 1,4-dioxane and slowly added dropwise to it, reacted overnight in ice bath for 1 hour; after the reaction, dilute with distilled water, extract the solution with ethyl acetate, and collect the lower water layer; adjust the pH of the water layer to pH=1 with HCl; Then extract the aqueous layer with ethyl acetate, collect the upper organic layer; evaporate the organic solvent to obtain the product Fmoc-γ-Mba, MS collection of spectra ( figure 2 ) to confirm its generation.
Embodiment 2
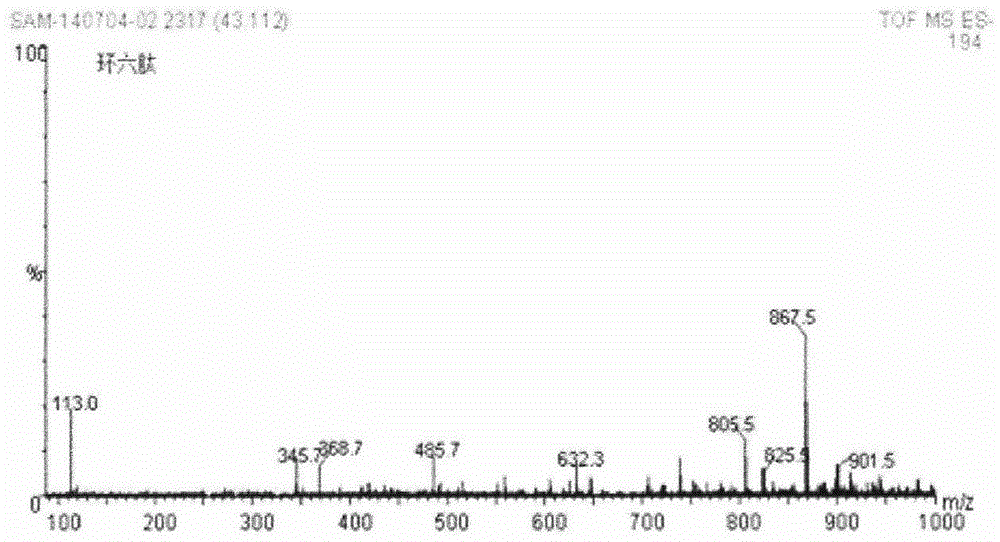
[0024] Example 2 Solid phase synthesis of cyclohexapeptide [cyclo-(L-Phe-γ-Mba-L-Phe-D-Phe-L-Phe-D-Phe)]
[0025] (1) Trityl chloride resin (TCR, 1.66mmol, 0.830g) was swollen with 8ml of dichloromethane (DCM) for 45min, and the solvent was removed by vacuum; then Fmoc-L-Phe (2.49mmol, 0.964g) and N, N-diisopropylethylamine (DIEA, 2.49mmol, 0.434ml) were dissolved in 10ml of dichloromethane and then added to the above resin, stirred and reacted at room temperature for 4h; (DCM / MeOH / DIEA=8 / 2 / 1, volume ratio) was capped, washed with DCM and anhydrous ether respectively, and finally vacuum-dried at 35° C. for 12 h.
[0026] (2) The resin (0.72 mmol, 1.6 g) dried in the previous step was swelled with 20 ml of DCM for 45 min, and the solvent was removed.
[0027] (3) The resin was washed with N,N-dimethylformamide (DMF), deprotected with 20% piperidine (PIP) in DMF, washed with DMF, and the solvent was removed.
[0028] (4) Fmoc-γ-Mba (3.96mmol, 1.476g) and 1-hydroxybenzotriazole...
Embodiment 3
[0036] Example 3 Cyclic hexapeptide self-assembled nanotubes
[0037] Accurately weigh 1mg of the above-mentioned cyclic hexapeptide, add 1ml of DCM, ultrasonically disperse, and let the cyclic hexapeptide self-assemble at room temperature for 2-96 hours. 35°C, 12h, the cyclohexapeptide self-assembled nanotubes were obtained, Figure 4 is its infrared spectrum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com