Preparation and application of fusion protein for treating hypercholesteremia
A technology of hypercholesterolemia and fusion protein, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of fusion protein
[0033] 1. Plasmid construction
[0034] 1.1 Gene and primer synthesis
[0035] The expressed sequence was recombined into the expression vector by the following synthetic gene and primers. The gene and primers were synthesized by Beijing Jinweizhi Company, a professional company, and the synthetic gene sequence was recombined into the plasmid vector pUC19, named pUC19-EGFwt, pUC19-mEGF 14 , pUC19-mEGF 16 and pUC19-3M EGF.
[0036] Synthetic fusion protein gene fragment:
[0037] EGFwt:
[0038] ATCACCGGTATGGGGCCCTGGGGCTGGAAATTGCGCTGGACCGTCGCCTTGCTCCTCGCCGCGGCGGGGACTGGGACCAACGAATGCTTGGACAACAACGGCGGCTGTTCCCACGTCTGCAATGACCTTAAGATCGGCTACGAGTGCCTGTGCCCCGACGGCTTCCAGCTGGTGGCCCAGCGAAGATGCGAAGGCGGCGGCGGCTCCGGAGGAGGAGGATCCGGAGGAGGAGGATCCGACAAAACTCACACATGCCCAC
[0039] mEGF 14 :
[0040] ATCACCGGTATGGGGCCCTGGGGCTGGAAATTGCGCTGGACCGTCGCCTTGCTCCTCGCCGCGGCGGGGACTGGGACCAACGAATGCTTGGACAACAACGGCGGCTGTTCCTACGTCTGCAATGACCTTAAGATCGGCTACGAGTG...
Embodiment 2
[0070] Embodiment 2 fusion protein bioactivity assay
[0071]The binding affinity of EGF-Fc fusion protein to PCSK9 was measured by biomembrane interferometry on Otect QK (Fortebio). The SA sensor sensor (Fortebio, product number 18-5063) was immobilized in PCSK9 containing 0.5% BSA and 1mM CaCl2 in TrisHCl pH 7.4 buffer, washed in the same buffer, and transferred to the buffer containing the same buffer. The concentration of 0-500 nM EGF-FC fusion protein in the wells. Signals for reference wells containing buffer only were subtracted from all binding data. Affinity KD was obtained by fitting to a steady state algorithm using Octet software. The determined KD values summarized in Table I show a 15- to 50-fold increase in affinity for mEGF-Fc mutant and 3mEGF-FC tandem mutant fusion proteins compared to EGFwt-Fc.
[0072] Table I, Binding affinity of EGFwt-Fc, mEGF-Fc mutants and 3mEGF-FC tandem mutations to PCSK9. KD values were determined by fitting the data to a ste...
Embodiment 3
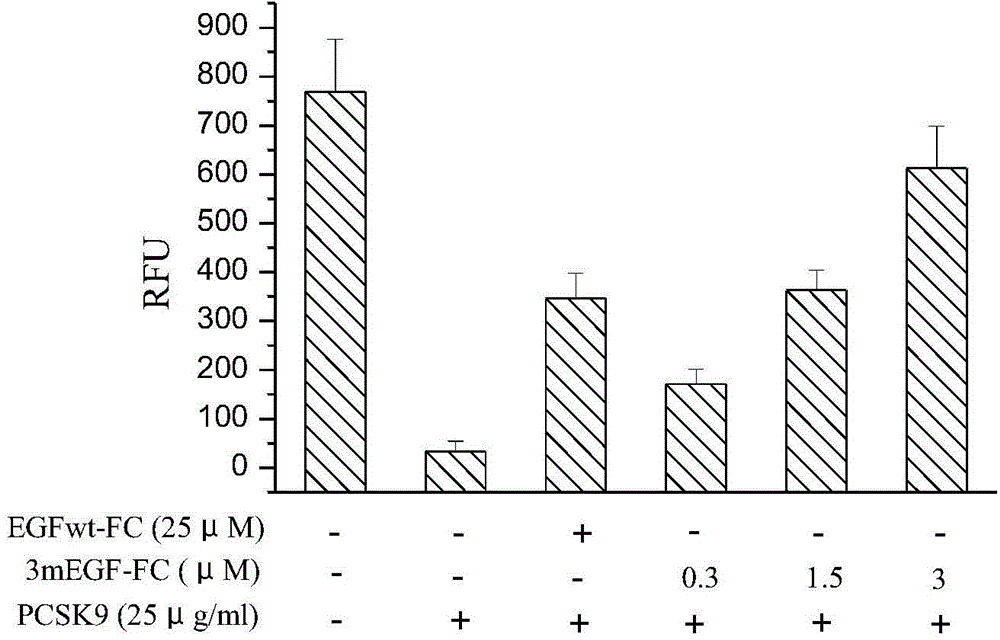
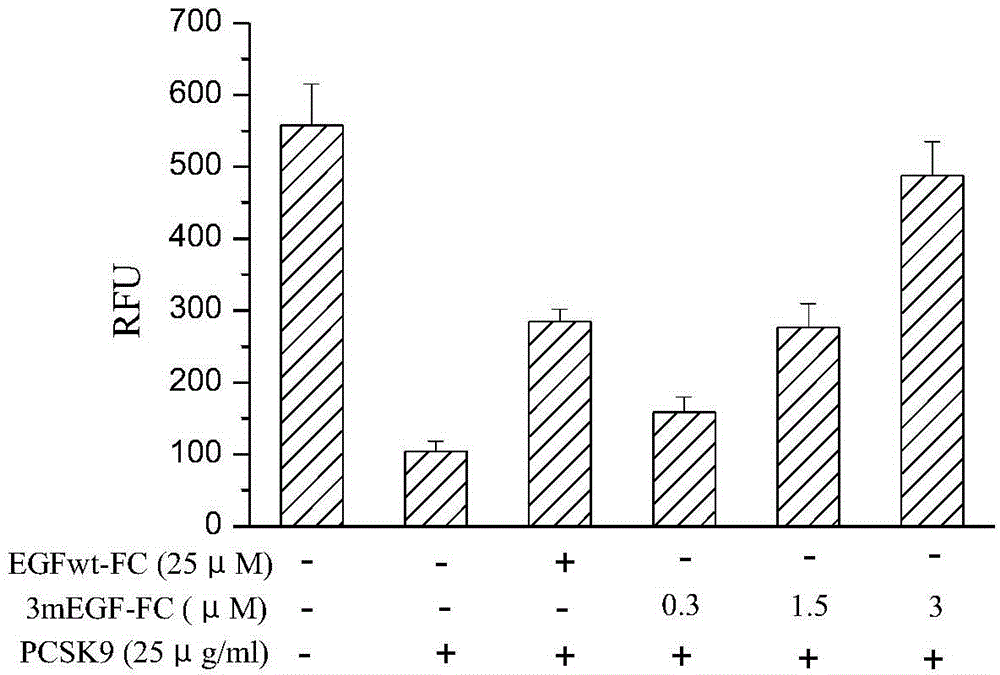
[0074] Embodiment 3 fusion protein bioactivity assay
[0075] Human liver cancer HepG2 cells were cultured to the logarithmic growth phase, the cells were washed once with PBS, digested with trypsin to make a cell suspension, and the cell density was adjusted to 1×10 6 cells / ml. The cells were seeded in a 96-well culture plate (100 μl / well), and 200 μl of medium was added to the edge wells. Place the culture plate at 37°C, 5% CO 2 In the incubator, continue to cultivate for 24 hours. Remove the cell culture supernatant, add 100 μl serum-free cell culture medium to each well, and continue culturing in the CO2 incubator for 16 hours; discard the supernatant, replace with serum-free cell culture medium again, add PCSK9 (final concentration 25 μg / ml), and add EGFwt-FC (10-50μM) or 3mEGF-Fc (03-3μM) was incubated at 37°C in a 5% CO2 incubator for 4 hours; (The final concentration is 6 μg / ml) and incubate for 3 hours, remove the supernatant, wash the cells with PBS 3 times, pla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com