CHO (Chinese hamster ovary) cell culture technology capable of reducing content of acidic variants
A culture process and cell culture technology, which is applied in the field of cell culture, can solve the problems of charge heterogeneity, affecting the validity period, affecting antibody activity, etc., and achieve the effects of improving quality, easy operation, and increasing antibody expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Cell line A was used to express and culture recombinant human tumor necrosis factor receptor-Fc recombinant protein (rhTNFR-Fc) antibody
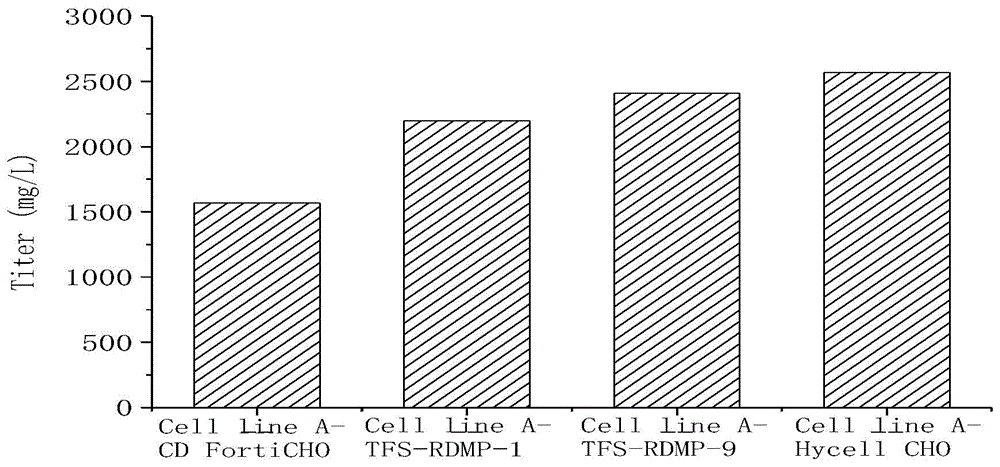
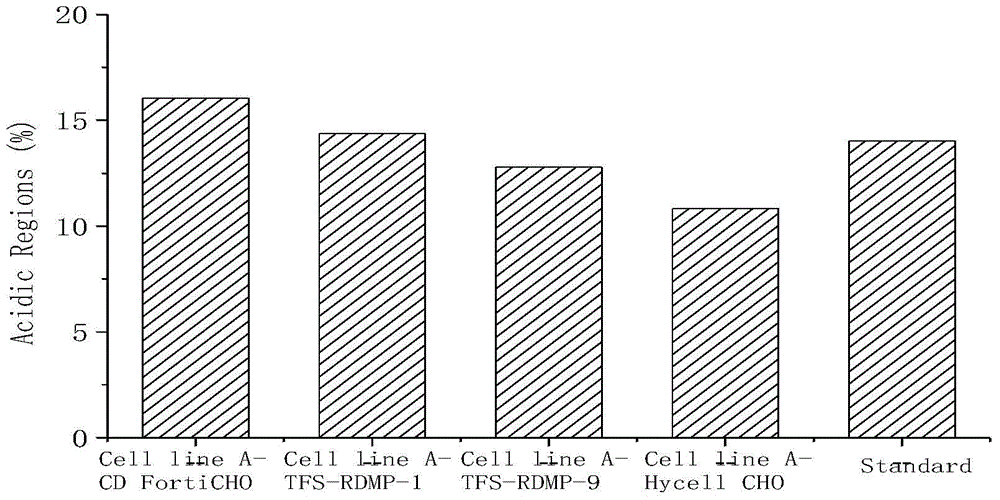
[0040] Table 1, Table 2, figure 1 and figure 2Cell line A-CD FortiCHO, Cell line A-TFS-RDMP-1, Cell line A-TFS-RDMP-9 and Cell line A-Hycell CHO refer to CD FortiCHO basal medium with 50% of the total volume of basal medium For inoculation, add CD FortiCHO basal medium or TFS-RDMP-1 basal medium or TFS-RDMP-9 basal medium or Hycell CHO with 25% of the total volume of the basal medium on the first day and the second day of culture respectively Basal medium.
[0041] 2. Optimization and screening of concentrated medium fed batch experiment methods
[0042] Concentrated Medium Feed Batch Experimental Method: Add 10% CHO CD Efficient Feed on the 3rd day of culture TM A and CHO CD Efficient Feed TM Mixture of B (the volume ratio of the two is 1:1), add 12.5% CHO CD Efficient Feed on the 5th day TM A. CHO CD Efficient Feed ...
Embodiment 2
[0050] 1. Cell line B was used for expression and culture of recombinant human tumor necrosis factor receptor-Fc recombinant protein (rhTNFR-Fc) antibody
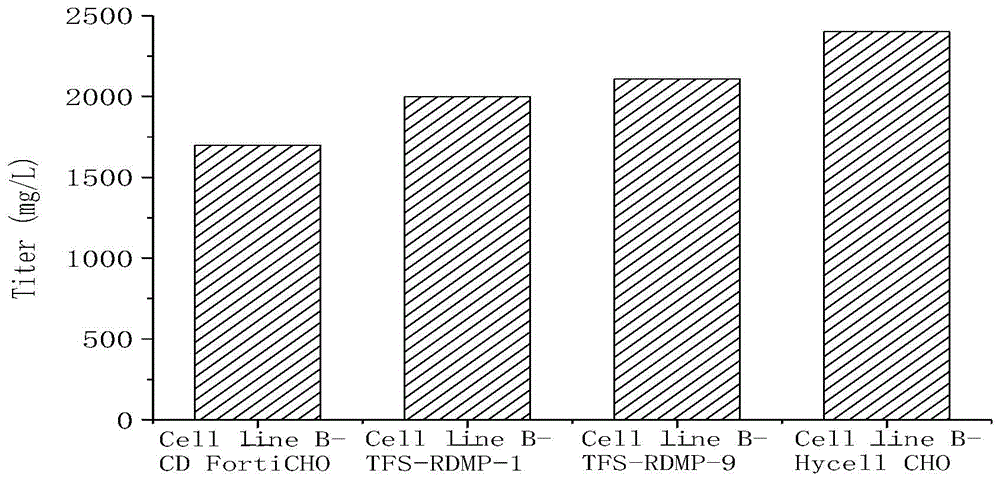
[0051] Table 3, Table 4, image 3 and Figure 4 Cell line B-CD FortiCHO, Cell line B-TFS-RDMP-1, Cell line B-TFS-RDMP-9 and Cell line B-Hycell CHO refer to CD FortiCHO basal medium with 50% of the total volume of basal medium For inoculation, add CD FortiCHO basal medium or TFS-RDMP-1 basal medium or TFS-RDMP-9 basal medium or Hycell CHO with 25% of the total volume of the basal medium on the first day and the second day of culture respectively Basal medium.
[0052] According to the method of Example 1, cell line B was used to express recombinant human tumor necrosis factor receptor-Fc recombinant protein (rhTNFR-Fc) antibody.
[0053] The cell viability and viable cell density of cell line B in different basal medium feeding methods are shown in Tables 3 and 4, in which different basal medium feeding methods have no si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com