GLP-1 derivative and preparation method and application thereof
A technology of GLP-1 and derivatives, applied in chemical instruments and methods, drug combinations, pharmaceutical formulations, etc., can solve problems such as difficulty in maintaining secondary structure and instability of GLP-1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
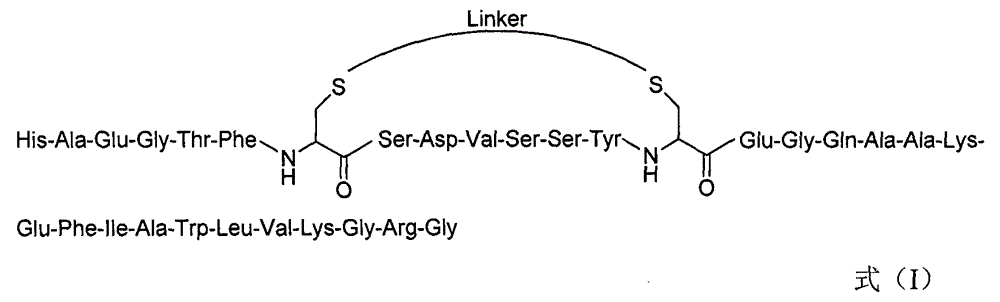
[0082] Embodiment 1: the synthesis of GLP-1 derivative Ia
[0083] (1) Synthesis of Cys-substituted linear peptides
[0084] Weigh 1 g of Wang resin with a substitution degree of 0.6 mmol / g in a solid phase reactor, add DCM to swell for 20 min, then add 20% PIP / DMF solution to react for 20 min to remove the Fmoc protecting group, wash with DCM, MeOH and DMF respectively Time, drain. Add Fmoc-Gly-OH (0.36g, 1.2mmol), HOBt (0.19g, 1.44mmol), DIC (0.23mL, 1.44mmol), DMF (5mL) into the solid phase reactor, and react at 25°C for 1h. The resin was washed and dried to obtain Fmoc-Gly-Wang resin, and the measured resin substitution degree was 0.5 mmol / g. Add 4 mL of blocking reagent (acetic anhydride (mmol): DIPEA (mmol) = 1:1) to the resin, react for 2.5 h, block the remaining amino groups, and use DCM (1 time), MeOH (1 time) and DMF (3 times) washing. Add 20% PIP / DMF solution and react for 20 minutes to remove the Fmoc protecting group, wash with DCM (once), MeOH (once) and DMF ...
Embodiment 2
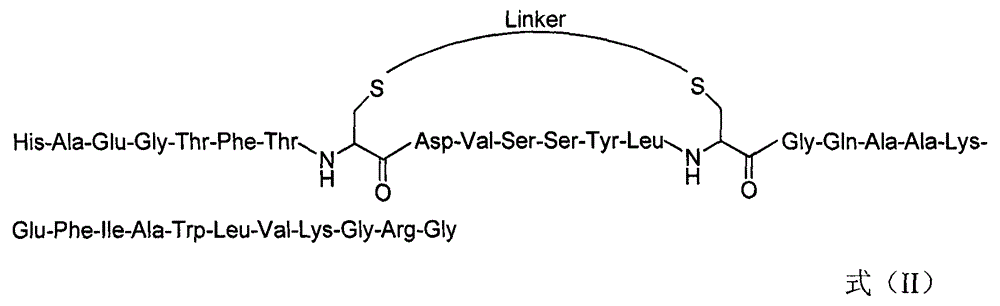
[0089] Example 2: Synthesis of GLP-1 derivative IIIb
[0090] (1) Synthesis of Cys-substituted linear peptides
[0091] With step (1) of embodiment 1, the only difference is that the Cys replacement position and amino acid are Asp 15 and Gly 22 .
[0092] (2) Synthesis of GLP-1 derivative IIIb
[0093] Weigh 0.1 g of the Cys-substituted linear polypeptide obtained in step (1), place it in a 50 mL round bottom flask, add 8 mL of acetonitrile aqueous solution (acetonitrile: water = 2: 1) to dissolve, and adjust the pH to 8 with saturated ammonium bicarbonate solution -9; 150 mg of 4,4'-bis(bromomethyl)diphenylamine dissolved in 3 mL of acetonitrile was added into a round-bottomed flask, stirred for 1 h, and 0.3 mL of acetic acid was added to quench the reaction, and the obtained product was separated and purified by HPLC. Freeze-dry to obtain GLP-1 derivative IIIb.
Embodiment 3
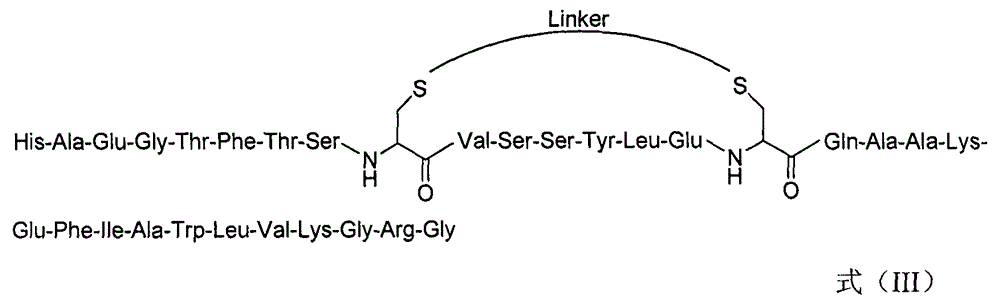
[0094] Example 3: Synthesis of GLP-1 derivative IXc
[0095] (1) Synthesis of Cys-substituted linear peptides
[0096] With embodiment 1 step (1), the only difference is that the Cys replacement position and amino acid are Ala 24 and Trp 31 .
[0097] (2) Synthesis of GLP-1 derivative IXc
[0098] Weigh 0.1 g of the Cys-substituted linear polypeptide obtained in step (1), place it in a 50 mL round bottom flask, add 8 mL of acetonitrile aqueous solution (acetonitrile: water = 2: 1) to dissolve, and adjust the pH to 8 with saturated ammonium bicarbonate solution -9; Add 120 mg of N,N'-(oxybis(4,1-phenylene))bis-(2-bromoacetamide) dissolved in 3 mL of acetonitrile into a round bottom flask, stir for 0.5 h, add 0.3 mL of acetic acid to quench reaction, the obtained product was separated and purified by HPLC, and freeze-dried to obtain the GLP-1 derivative IXc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com