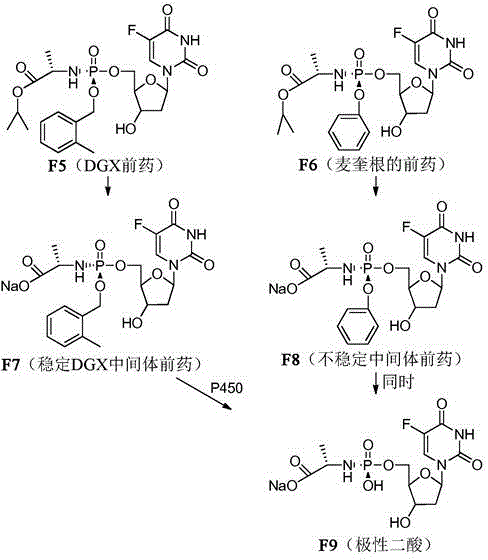
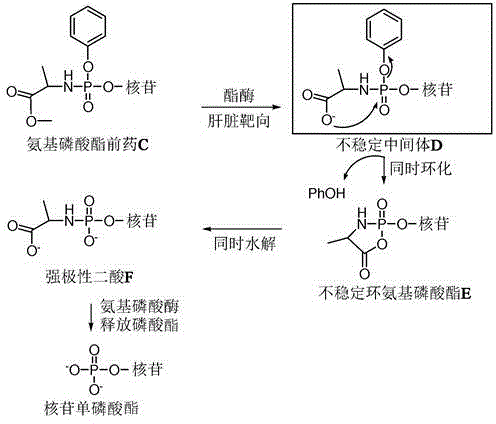
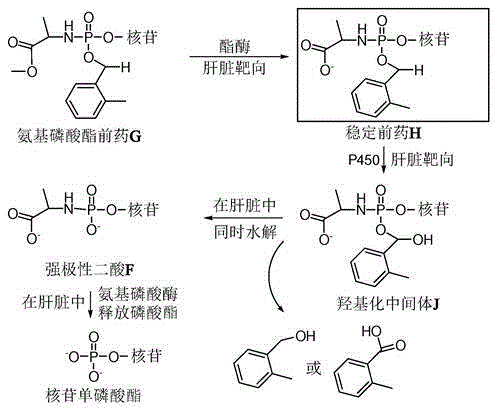
Double-liver-targeting phosphoramidate and phosphonoamidate prodrugs
A prodrug and pharmacy technology, applied in the field of double liver targeting phosphoramidate or aminophosphonate prodrug, can solve the problem of unreported biological activity, inability to effectively cleave active nucleoside phosphate, and unproven biological applicability And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0248] Example 1. Preparation of monochlorophosphoramidate 3.
[0249]
[0250] To a solution of phosphorus oxychloride (3.07 g, 20 mmol) in THF (40 mL) was added 2-methylbenzyl alcohol (1, 2.44 g, 20 mmol) and triethylamine (2.02 g, 20 mmol) at -78°C. THF (10 mL), and the mixture was stirred at -78°C for 3 hours. At -78°C, L-alanyl isopropyl ester hydrochloride (3.35 g, 20 mmol) and THF (10 mL) containing triethylamine (4.04 g, 40 mmol) were successively added to the resulting mixture, and at -78 The mixture was stirred at °C for 1 hour, then at room temperature overnight to obtain a crude compound 3 reaction mixture.
example 2
[0251] Example 2. Similarly, benzyl monochlorophosphoramidate 3' was prepared.
[0252]
example 3
[0253] Example 3. Preparation of Chiral Reagent 4 as a Diastereomerically Enriched Isomer.
[0254]
[0255] As in Example 1, a mixture of compound 3 was prepared on a 20 mmol scale. A solution of pentafluorophenol (20 mmol) and triethylamine (20 mmol) was added to the mixture. To the mixture was added another portion of triethylamine (20 mmol) and the mixture was stirred at room temperature for 4 hours. EtOAc (200 mL) was added and the mixture was washed with water and brine, and washed with Na 2 SO 4 dry. The solvent was removed and the residue was purified by silica gel column chromatography (5-50% EtOAc in hexanes) to give the crude compound as a mixture of diastereoisomers. 1 H-NMR (CDCl 3 ):δ7.18-7.36(m,4H),5.23(dd,J=7.2Hz,1H),5.02(m,1H),4.00(m,1H),3.76(m,1H),2.38,2.37( ss, 3H), 1.42, 1.37 (d, J=7.2Hz, 2H), 1.24 (m, 9H). Recrystallization of the mixture of diastereomers from EtOAc-hexane afforded diastereomerically enriched reagent 4. 1 H-NMR (CDCl 3):δ7.17-7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com