piRNA antisense nucleotide pharmaceutical composition and application thereof
A technology of antisense nucleotides and compositions, which is applied in the direction of drug combinations, pharmaceutical formulations, genetic material components, etc., can solve the problem that the pathogenesis of heart disease is not completely clear, and the prevention, diagnosis and treatment of heart disease cannot achieve satisfactory results. Effects and other issues, to achieve the effect of wide range of use, safe and reliable use, and friendly application environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The piRNA antisense nucleotide pharmaceutical composition includes piRNA antisense nucleotides and a pharmaceutically acceptable carrier; the piRNA antisense nucleotide sequence used is shown in SEQ ID NO: 1 in the sequence listing: 5'-CCUUGGCACAUGCGCAGAUUAUUUGUUUA- 3'; the carrier is one or more of chitosan, cholesterol, nanoparticles and liposomes, preferably liposome nanoparticles. The content of the piRNA antisense nucleotide in the pharmaceutical composition is 0.5-1 gram.
Embodiment 2
[0021] Example 2, detection of piRNA expression in myocardial ischemia and cardiomyocyte apoptosis induced by hypoxia
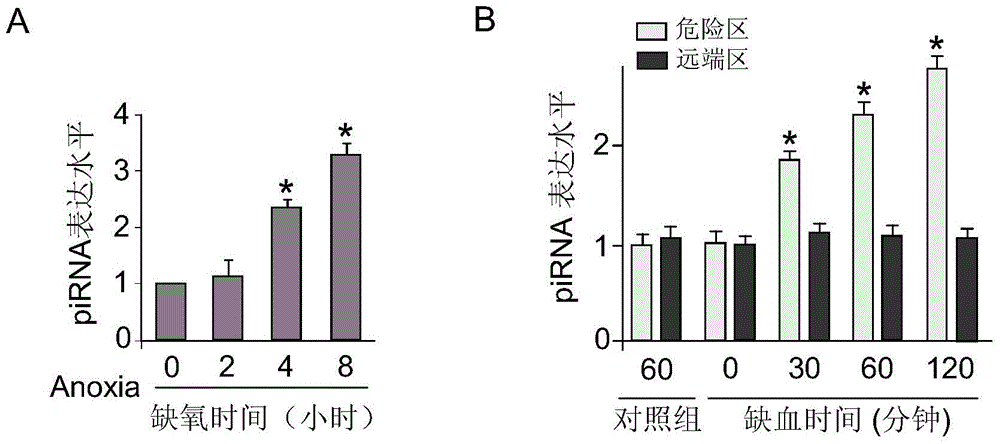
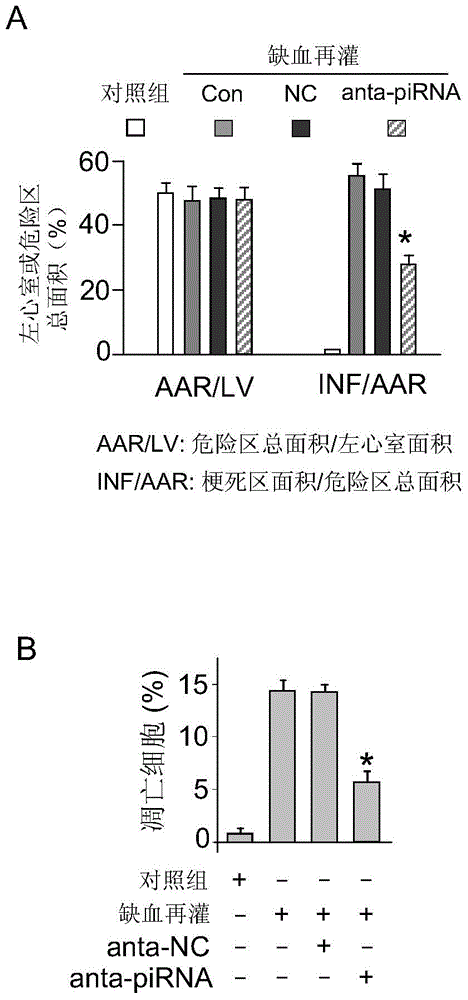
[0022] In this embodiment, the experimental model of cardiomyocyte apoptosis is cultivated by conventional methods to cultivate the primary cardiomyocytes of rat suckling rats, cultured in a hypoxic incubator (oxygen concentration lower than 1%) for different times, extracting RNA, and detecting by real-time fluorescent quantitative PCR technology piRNA expression levels, figure 1 It is a diagram of the change of piRNA expression level in myocardial cell apoptosis process of myocardial ischemia injury and hypoxia treatment (Anoxia), wherein figure 1 A shows the expression level of piRNA in primary cardiomyocytes under hypoxia treatment; figure 1 B shows the expression level of piRNA in myocardial ischemia injury in mice; it was significantly up-regulated within 4h-8h of hypoxia treatment ( figure 1 A); The myocardial ischemia model was established by ligati...
Embodiment 3
[0023] Embodiment 3, the experiment that piRNA antisense nucleotide inhibits cardiomyocyte apoptosis
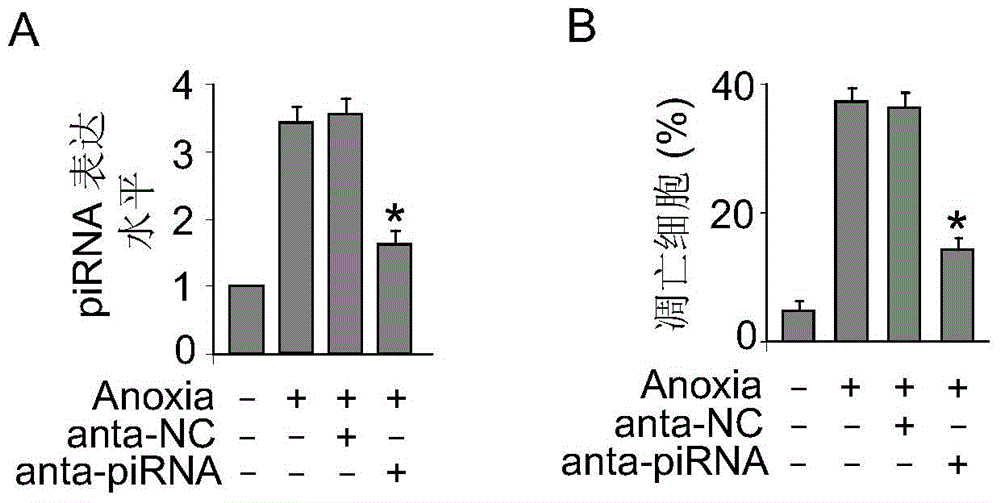
[0024] In this example, primary cultured cardiomyocytes were used as a model to transfect piRNA antisense nucleotides (the piRNA antisense nucleotide sequence is a completely reverse complementary sequence to the piRNA sequence, 5'-CCUUGGCACAUGCGCAGAUUAUUUGUUUA-3', combined with piRNA to inhibit the expression of piRNA), the transfection experiment method can be found in the aforementioned literature, after 24 hours of transfection, the treated cells were cultured in a hypoxic incubator for 3 hours to induce cell apoptosis, and trypan blue staining method was used to detect cardiomyocyte apoptosis death, see figure 2 ,in figure 2 A represents primary cardiomyocytes transfected with piRNA antisense nucleotides (anta-piRNA) to inhibit the expression of endogenous piRNA; figure 2 B represents the effect on hypoxia-induced cardiomyocyte apoptosis, and NC was transfected as a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com