Preparation method and application of amphiphilic photoelectric active branched macromolecules
A photoelectric active, macromolecular technology, applied in the direction of electrochemical variables of materials, can solve the problems of easy entanglement and agglomeration, limited application, poor solubility, etc., and achieves easy preparation and operation, excellent product performance, and controllable polymer structure. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
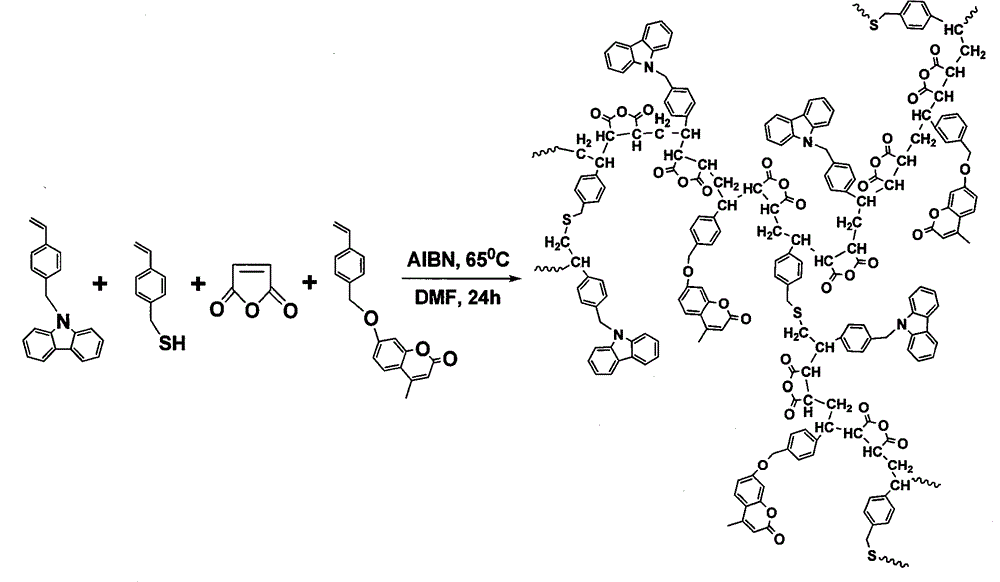
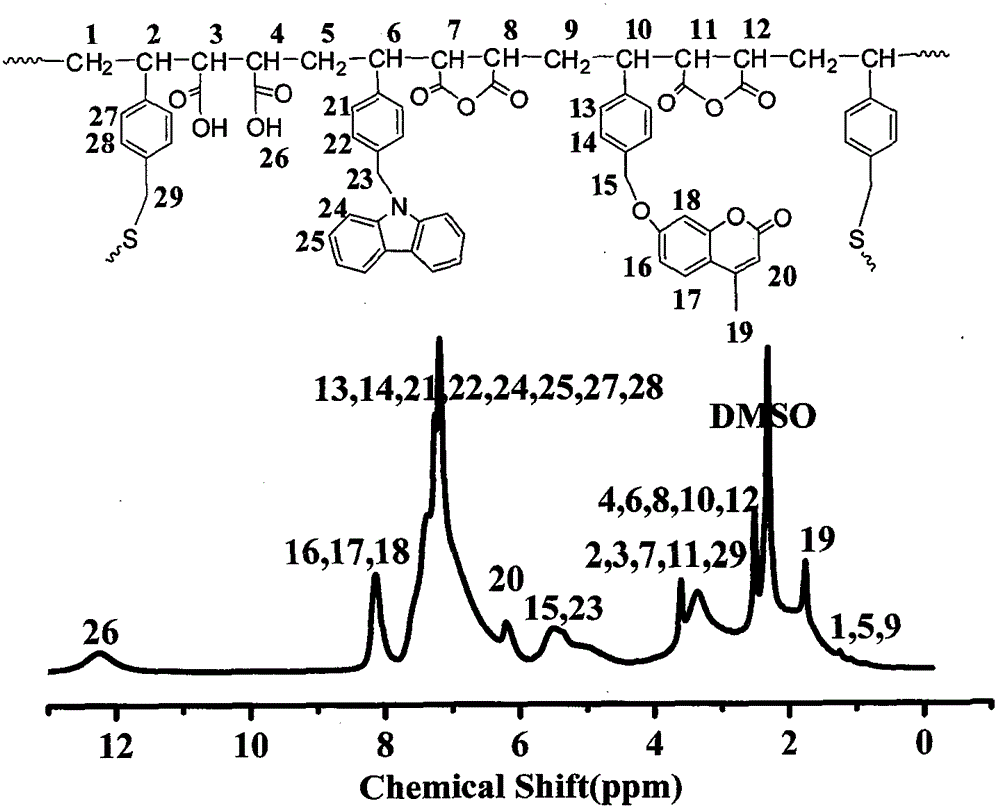
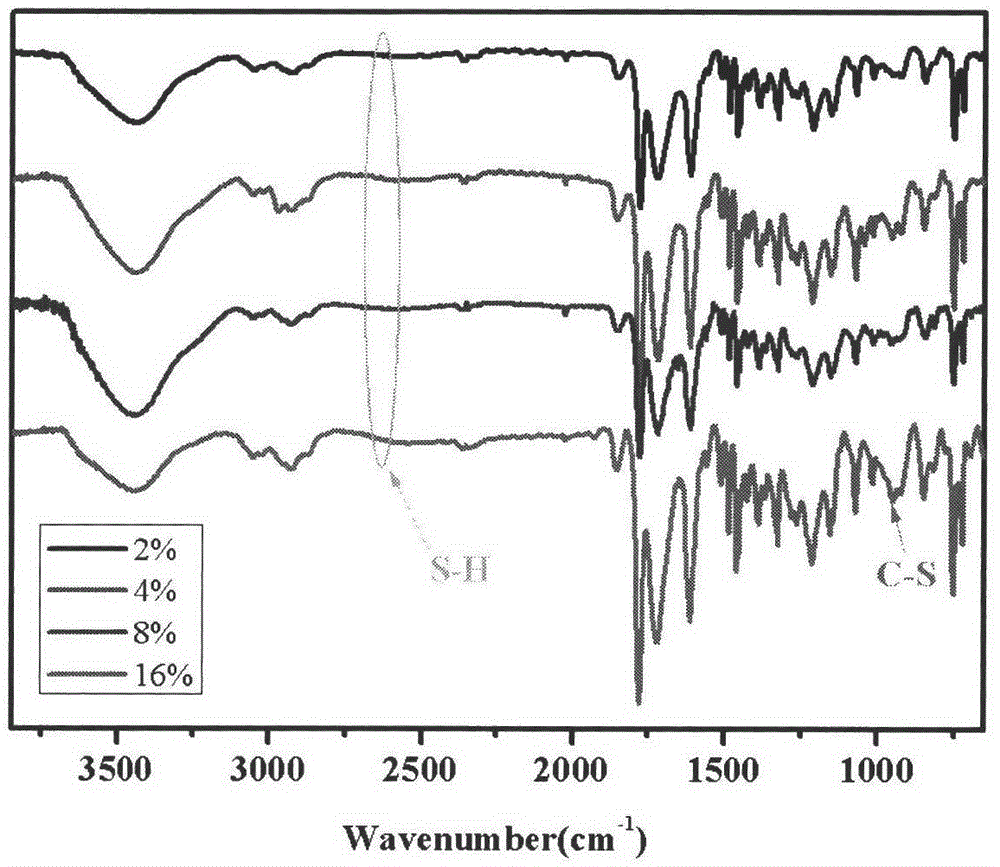
[0024] 1.46g (5mmol) 7-(4-vinylbenzyloxy)-4-methylcoumarin, 1.42g (5mmol) N-(4-vinylbenzyl)-9H-carbazole, 1.27g (13mmol) maleic anhydride, 0.069g (0.46mmol) 4-vinylbenzylmercaptan, 0.077g (0.469mmol) azobisisobutyl Nitrile, dissolved in 40ml of N,N-dimethylformamide polymerization solvent, heated the flask in a constant temperature oil bath, stirred until it was completely dissolved, fed nitrogen into the flask for 30 minutes, raised the temperature to 65°C, and reacted at constant temperature for 24 hours. After the reaction is finished, cool to room temperature, precipitate the reaction product in toluene, and obtain a white powdery solid by suction filtration. After repeated dissolution, precipitation, and suction filtration three times, dry in a vacuum oven to constant weight to obtain amphiphilic photoactive branched macromolecules product.
Embodiment 2
[0026] 1.46g (5mmol) 7-(4-vinylbenzyloxy)-4-methylcoumarin, 1.42g (5mmol) N-(4-vinylbenzyl)-9H-carbazole, 1.27g (13mmol) maleic anhydride, 0.138g (0.92mmol) 4-vinylbenzylmercaptan, 0.078g (0.478mmol) azobisisobutyl Nitrile, dissolved in 40ml of N,N-dimethylformamide polymerization solvent, heated the flask in a constant temperature oil bath, stirred until it was completely dissolved, fed nitrogen into the flask for 30 minutes, raised the temperature to 65°C, and reacted at constant temperature for 24 hours. After the reaction is finished, cool to room temperature, precipitate the reaction product in toluene, and obtain a white powdery solid by suction filtration. After repeated dissolution, precipitation, and suction filtration three times, dry in a vacuum oven to constant weight to obtain amphiphilic photoactive branched macromolecules product.
Embodiment 3
[0028]1.46g (5mmol) 7-(4-vinylbenzyloxy)-4-methylcoumarin, 1.42g (5mmol) N-(4-vinylbenzyl)-9H-carbazole, 1.27g (13mmol) maleic anhydride, 0.276g (1.84mmol) 4-vinylbenzylmercaptan, 0.082g (0.497mmol) azobisisobutyl Nitrile, dissolved in 40ml of N,N-dimethylformamide polymerization solvent, heated the flask in a constant temperature oil bath, stirred until it was completely dissolved, fed nitrogen into the flask for 30 minutes, raised the temperature to 65°C, and reacted at constant temperature for 24 hours. After the reaction is finished, cool to room temperature, precipitate the reaction product in toluene, and obtain a white powdery solid by suction filtration. After repeated dissolution, precipitation, and suction filtration three times, dry in a vacuum oven to constant weight to obtain amphiphilic photoactive branched macromolecules product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com