Factor VIII complex with XTEN and von willebrand factor protein, and uses thereof
A technology of hemophilia factor and FVIII, which is applied in the direction of expression enhancement stability/folded protein fusion, factor VII, blood coagulation/fibrinolysis factor, etc., and can solve problems such as half-life increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
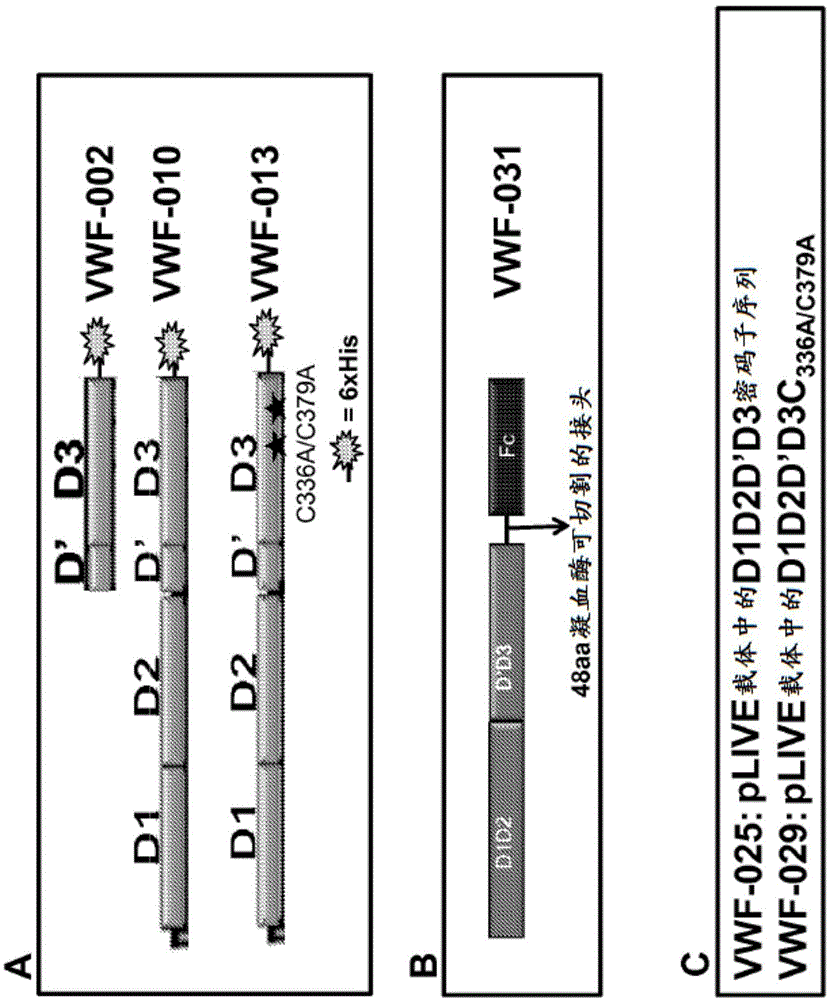
[0581] Example 1: Cloning of different VWF domains (Figure 1)
[0582] (a) Clone pSYN-VWF-002
[0583] pSYN-VWF-002 contains the nucleotide sequence encoding the VWF fragment, which is amino acids 1-477 of SEQ ID NO:100. [VWF-D'D3 protein sequence] Amino acid numbering indicates the mature VWF sequence without pro-peptide and corresponds to amino acids 764-1240 of SEQ ID NO:2. The pSYN-VWF-002 construct has a FVIII signal peptide at the N-terminus, which allows proper secretion of the synthetic protein and a subsequent 6xHis tag at the C-terminus (for protein purification). It was synthesized by using the following primer combinations:
[0584] ESC48-Fwd-VWF-D'D3 with VIII signal and BsiW1 site
[0585] TCGCGACGTACGGCCGCCACCATGCAAATAGAGCTCTCACCTGCTTCTTTCTGT
[0586] GCCTTTTGCGATTCTGCTTTAGCCTATCCTGTCGGCCCCCCATG (SEQ ID NO:
[0587] 90)
[0588] ESC51-Rev-VWF D'D3 with 6His and Not 1 sites (1-477 amino acids)
[0589] TGACCTCGAGCGGCCGCTCAGTGGTGATGGTGATGATGCGGCTCCTGGCAGGCT...
Embodiment 2
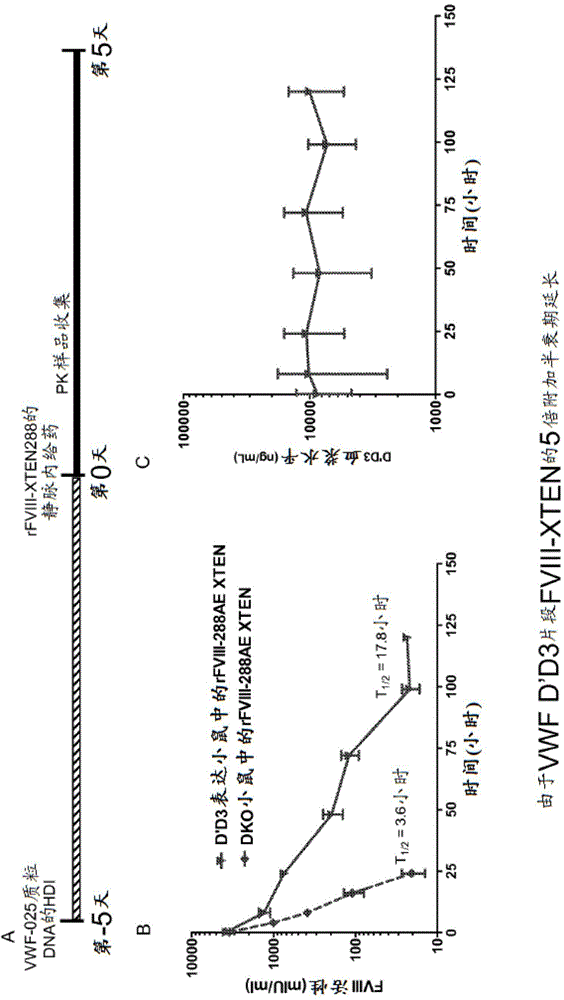
[0622] Example 2: Effect of D'D3 and XTEN fusions on FVIII half-life extension
[0623] To evaluate the D'D3FVIII half-life extension potential of rFVIII-XTEN fusion proteins, VWF D'D3 dimers were introduced into FVIII-VWF DKO mice by hydrodynamic injection of their corresponding DNA construct VWF-025 (Example 1) . After D'D3 had reached steady-state expression (day 5 post-injection), a single dose of rFVIII-XTEN was administered by intravenous injection at a dose of 200 IU / kg. Blood samples were collected up to 120 hours after rFVIII-XTEN administration. Plasma FVIII activity was analyzed by FVIII chromogenic assay. D'D3 expression levels were measured by VWF ELISA, and rFVIIIFc PK curves were analyzed using the WinNonlin program.
[0624] The results of the study are shown in Figure 2 and the PK parameters of rFVIII-XTEN with / without D'D3 in circulation are listed in Table 16. D'D3 dimer further prolongs rFIII-XTEN t 1 / 2 , increased 5 times from 3.4 hours to 17.8 hours....
Embodiment 3
[0635] Example 3: Plasmid Construction of XTEN Containing FVIII / VWF Constructs
[0636] (a) Clone pSYN-FVIII-161 (Figure 3)
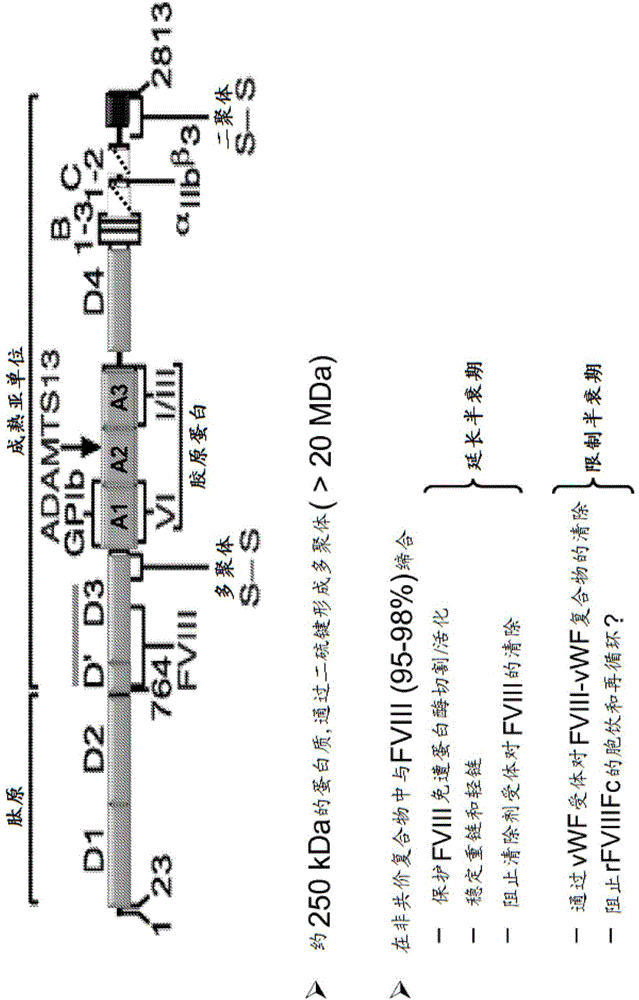
[0637] The FVIII-161 plasmid contains a single-chain Fc (scFc) scaffold with an enzymatic cleavage site that is processed in the cell during synthesis. The construct has the FVIII binding domain (D'D3) of full length VWF.
[0638] A plasmid (pSYN-FVIII-161 ) was designed to express FVIII-Fc and VWF-Fc heterodimers in which the D'D3 domain binds to FVIII and prevents FVIII from interacting with phospholipids and activated protein C. Protein from pSYN-FVIII-161 is expressed in cells as a single polypeptide in which the C-terminus of the FVIII-Fc subunit is linked to VWF D'D3 via a 6x (GGGGS) polypeptide linker (SEQ ID NO: 64) - the N-terminus of the Fc subunit. In addition, RRRRS (SEQ ID NO: 11) and RKRRKR (SEQ ID NO: 10) sequences were inserted at the 5' and 3' ends of the polypeptide linker, respectively, for passage of the proprotein convertase foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com