A kind of benzoacridine derivative, its preparation method and its application
A technology of benzoacridine and derivatives, which is applied in the field of organic electroluminescence, can solve the problems of reducing the cost of OLEDs, disadvantages, and increasing the complexity of device manufacturing processes, etc., and achieves high glass transition temperature, good thermal stability, and good The effect of receiving electrons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
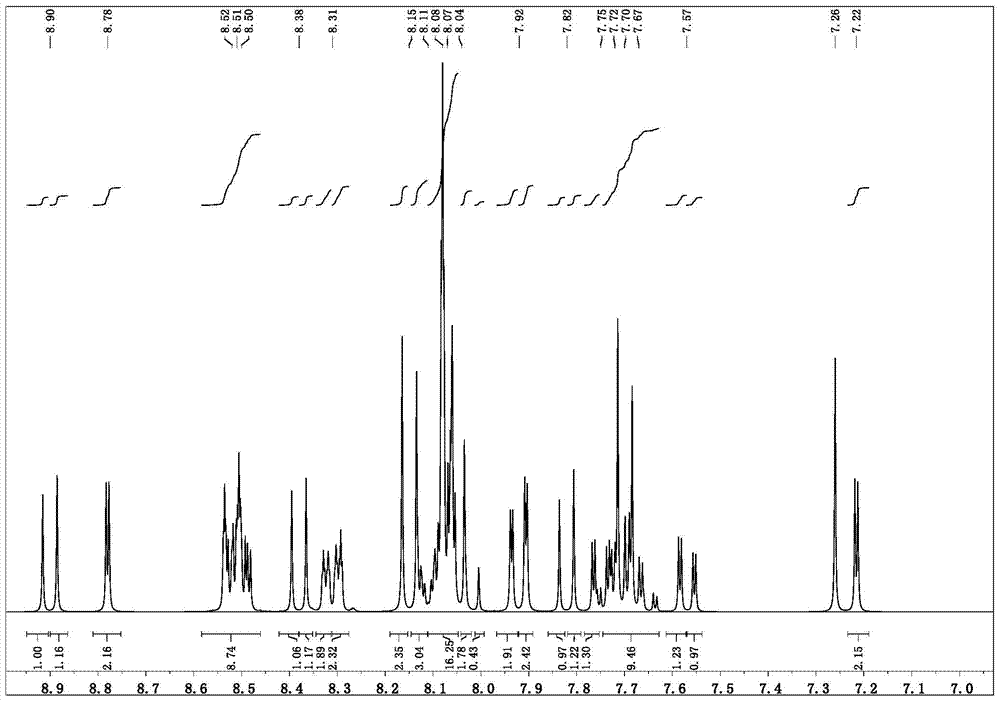
[0040]The synthesis of the parent 9-bromo-7-(4-bromophenyl)benzo[c]acridine (reference: Org.Biomol.Chem., 2010, 8, 326-330), the reaction route is as follows:
[0041]
[0042] N-(4-bromophenyl)-1-naphthylamine 23.8g (molecular weight 297, 0.08mol), 4-bromobenzoic acid 16g (molecular weight 200, 0.08mol), ZnCl 2 Use 16.1g (molecular weight: 134, 0.12mol) and heat in a sand bath for 5 hours while stirring at a temperature of 240-260°C. Cool, dissolve, mix with silica gel, and separate by column (eluent: dichloromethane / ethyl acetate=20:1) to obtain 18.81 g of product with a yield of 51% and a molecular weight of 463.
Embodiment 2
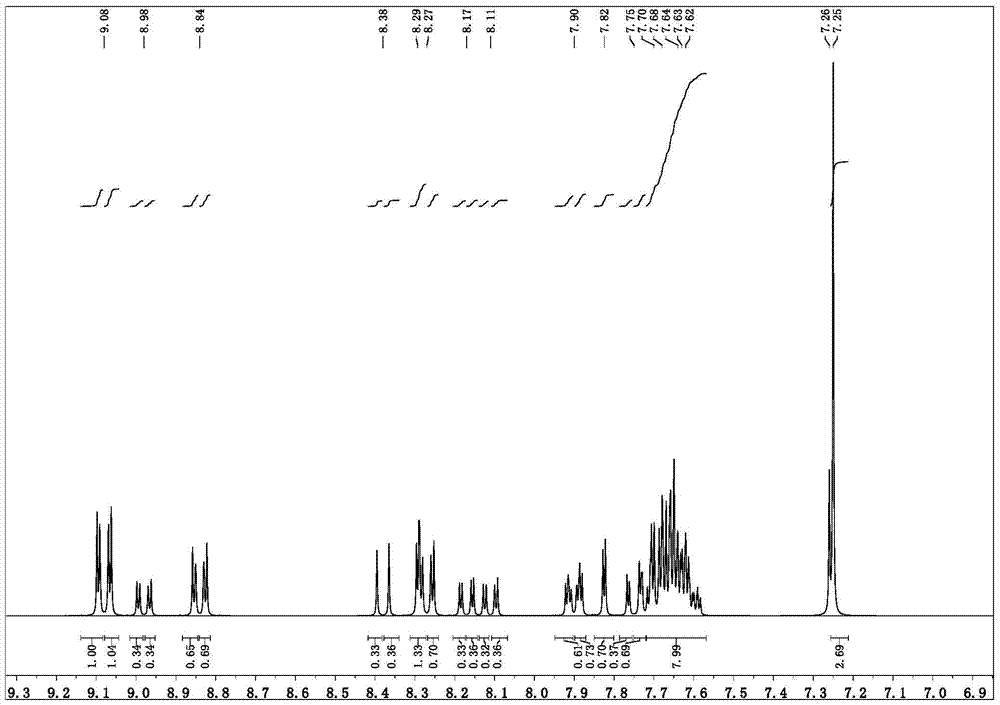
[0044] Synthesis of parent 9-bromo-7-(4-bromopyridin-2-yl)benzo[c]acridine
[0045] The synthesis steps are the same as the previous 1, except that benzoic acid is changed into 4-bromopyridine-2-carboxylic acid, other reagents and reaction conditions are unchanged, the reaction is completed, and column chromatography is separated to obtain the target parent 9-bromo-7-(4-bromo pyridin-2-yl)benzo[c]acridine. The reaction pathway is as follows:
[0046]
Embodiment 3
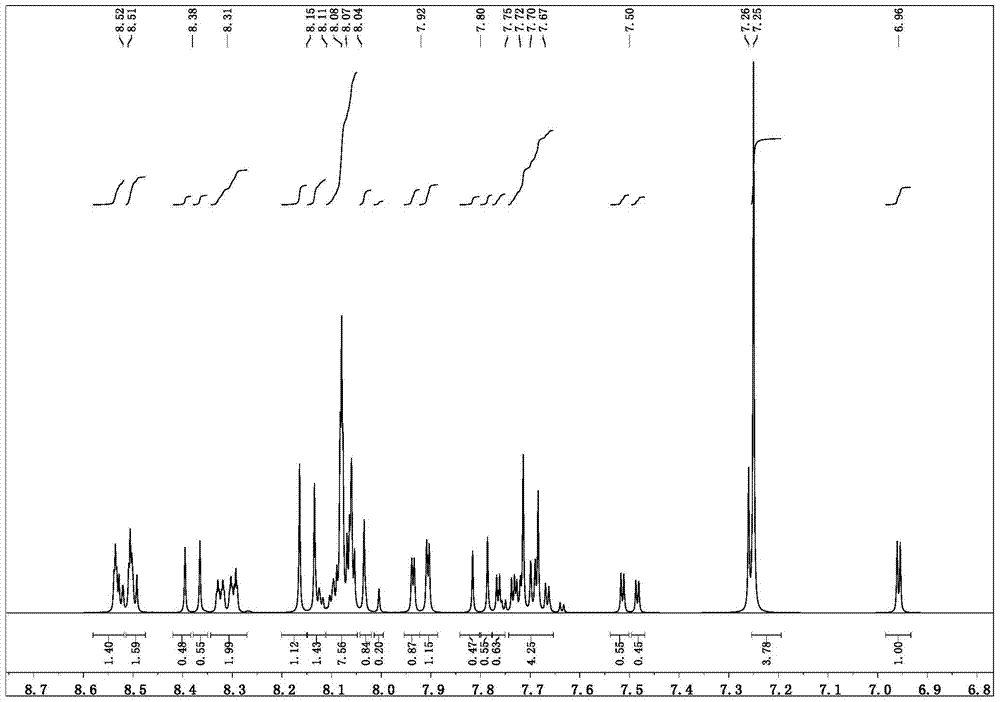
[0048] Synthesis of parent 9-bromo-7-(quinolin-2-yl)benzo[b]acridine
[0049] The synthesis steps are the same as the previous 1, except that benzoic acid is changed into quinoline-2-carboxylic acid, other reagents and reaction conditions are unchanged, the reaction is completed, and column chromatography is separated to obtain the target parent 9-bromo-7-(quinoline-2- base) benzo[b]acridine. The reaction pathway is as follows:
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com