A kind of phenazine derivative and its application in organic electroluminescence device
A technology for electroluminescent devices and derivatives, applied to its intermediates and its preparation, application in electron transport materials, and phenazine derivatives, which can solve the problems of increasing the complexity of device manufacturing processes, reducing OLED costs, disadvantages, etc. , to achieve good electronic acceptability, reduce power consumption, and reduce the effect of working voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
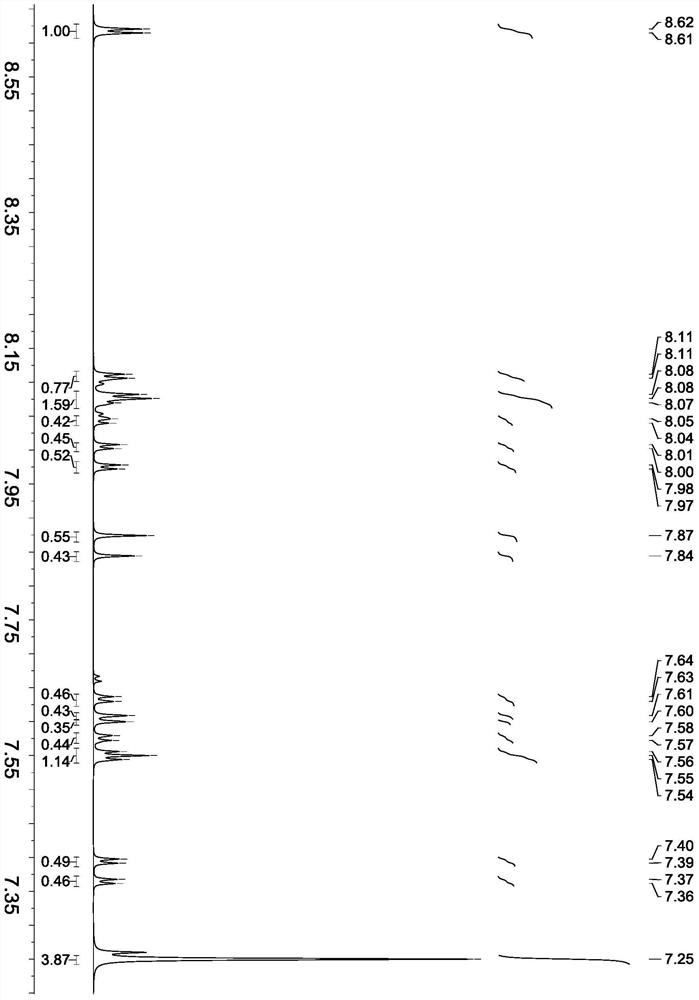
[0049] The synthesis of embodiment 1 dibromophenazine
[0050]
[0051] Add 3.91 grams of 4-bromo-o-phenylenediamine (molecular weight 186, 0.021 mol), 3.91 grams of 4-bromo-o-benzoquinone (molecular weight 186, 0.021 mol), ethanol (40 ml) into a 250 ml three-necked flask, and stir for 3 minutes Add 0.2 gram of concentrated sulfuric acid (concentration 98%) dropwise, and react at 65°C for 4 hours. After the reaction, cool to room temperature, filter, wash with water, dry, separate by column chromatography, and rinse with ethyl acetate / petroleum ether to obtain Almost equal amounts of 2,7-dibromophenazine and 2,8-dibromophenazine totaled 5.82 g (molecular weight: 336), and the total yield was 82.4%.
Embodiment 2
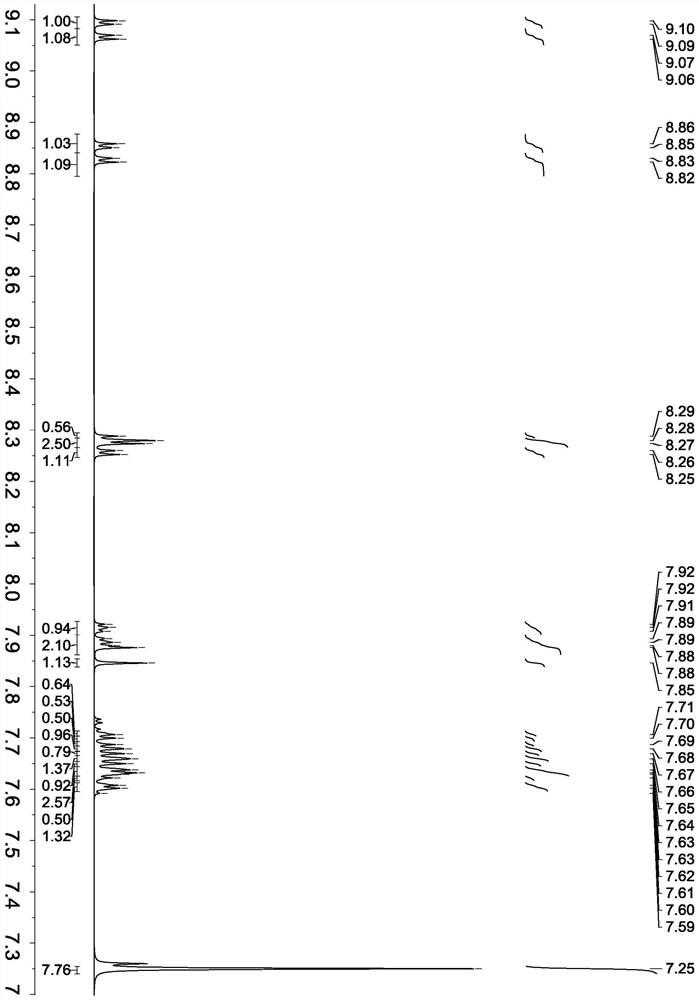
[0053] Synthesis of compound shown in formula (4)
[0054]
[0055] 1000 ml three-necked flask equipped with magnetic stirring and nitrogen protection, add 6.72 g of 2,8-dibromophenazine (molecular weight 336, 0.02 mol), 7.4 g of naphthalene-2-boronic acid (molecular weight 172, 0.043 mol), tetrakis(triphenyl 2.3 g of palladium (molecular weight: 1154, 0.002 mol), 100 ml of 2M sodium carbonate aqueous solution, 100 ml of toluene, and 100 ml of ethanol. After argon replacement, the reaction was refluxed, and the reaction was monitored by thin-layer chromatography (TLC). After 3.5 hours, TLC found that the raw material bromide was completely reacted, and only the product point was present. The temperature was lowered, the organic layer was separated, evaporated to dryness, separated by column chromatography, and washed with ethyl acetate / petroleum ether to obtain 8.1 g of the compound represented by formula (4), with a molecular weight of 432 and a yield of 93.8%.
[0056] P...
Embodiment 3
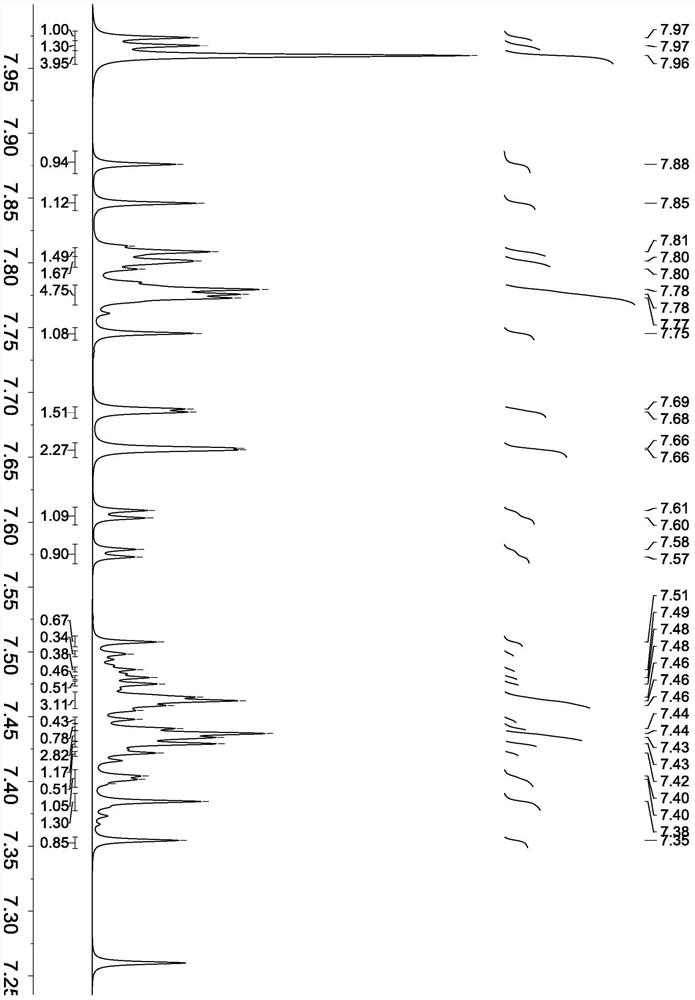
[0058] Synthesis of compound shown in formula (5)
[0059] The synthesis steps are the same as in Example 2, except that naphthalene-2-boronic acid is changed to naphthalene-1-boronic acid, and other reagents remain unchanged to obtain the compound shown in formula (5).
[0060] Product MS (m / e): 432, elemental analysis (C 32 h 20 N 2 ): theoretical value C: 88.86%, H: 4.66%, N: 6.48%; measured value C: 88.88%, H: 4.67%, N: 6.45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com