A kind of bibenzimidazole derivative, its preparation method and its application
A technology of benzimidazole and derivatives, applied in the field of organic electroluminescence, can solve the problems of low glass transition temperature, shortened device life, small molecular weight, etc., and achieves improved luminous efficiency, lower lighting voltage, and high glass state transition. effect of temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
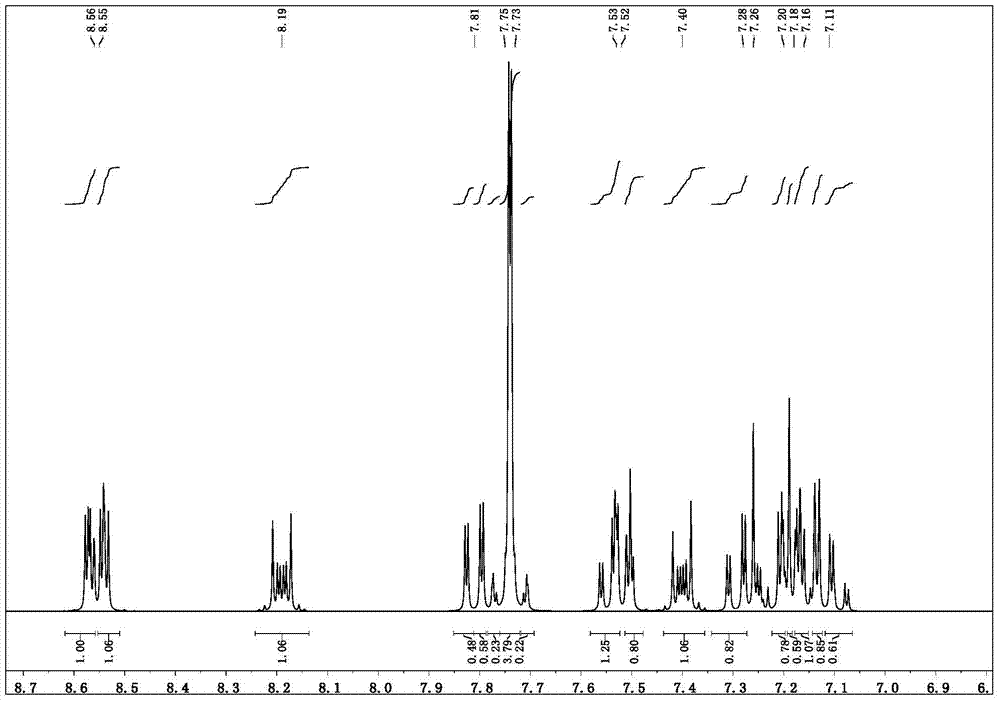
[0036] Synthesis of intermediate 1-(4-bromophenyl)-1H-benzo[d]imidazole
[0037]
[0038] 500 ml one-necked bottle, equipped with a magnetic stirrer, add 9.5 g of 1H-benzo[d] imidazole (molecular weight 118, 0.08 mol), p-bromoiodobenzene 28.2 g (molecular weight 282, 0.1 mol), CuI dosage 1.5 g (molecular weight 190, 0.0079mol), potassium carbonate 20.7g (molecular weight 138, 0.15mol), DMF 200ml, piperidine-2-carboxylic acid 2.1g (molecular weight 129, 0.016mol). After argon replacement, stir and reflux, monitor the reaction with TLC, the reaction is complete after 3 hours, lower the temperature, add 200ml of water, the product precipitates, filter, wash with water repeatedly, the product is finally purified by column chromatography, and eluted with ethyl acetate / petroleum ether to obtain 15g Compound 1-(4-bromophenyl)-1H-benzo[d]imidazole, molecular weight 272, yield 68.5%.
Embodiment 2
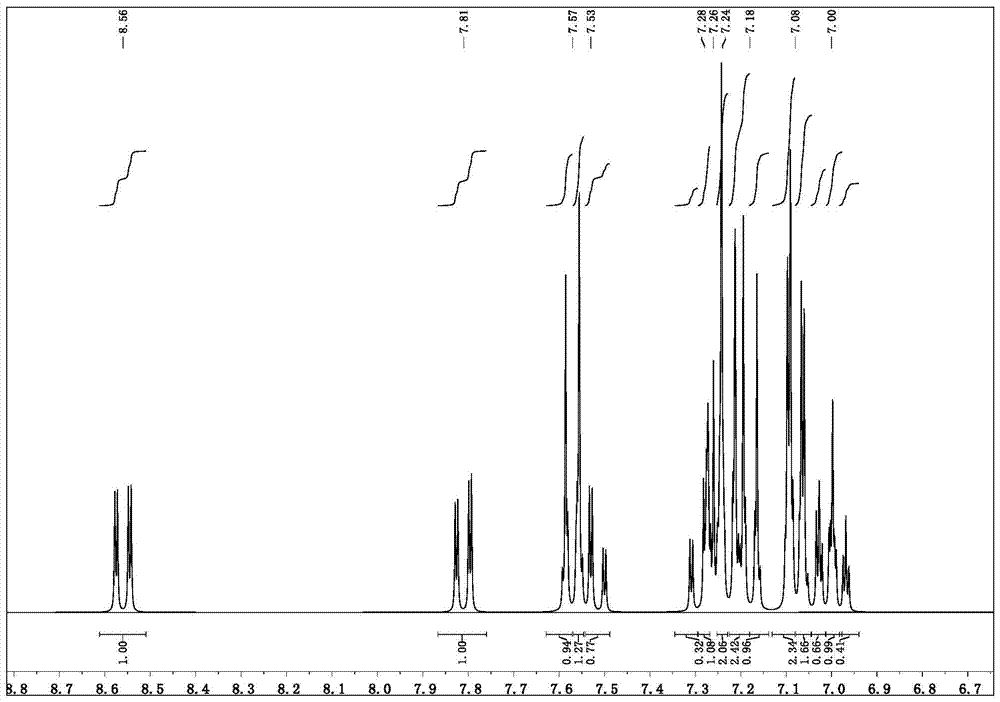
[0040]Synthesis of parent 1,1'-bis(4-bromophenyl)-1H,1'H-2,2'-bibenzo[d]imidazole (reference: Org.Biomol.Chem.,2010,8,326- 330)
[0041]
[0042] Add 1-(4-bromophenyl)-1H-benzo[d]imidazole 13.6g (molecular weight 272, 0.05mol), 200ml xylene, copper acetate 1.8g (molecular weight 181, 0.01mol) in a 500ml three-necked flask, Install the air duct, and air is slowly introduced during the reflux period, pay attention to adding xylene solvent, reflux and stir, monitor the reaction with TLC, the reaction is completed in about 12 hrs, and cool to room temperature. The xylene was distilled off and purified by column chromatography to obtain 11.9 g of a solid product with a molecular weight of 542 and a yield of 87.5%.
Embodiment 3
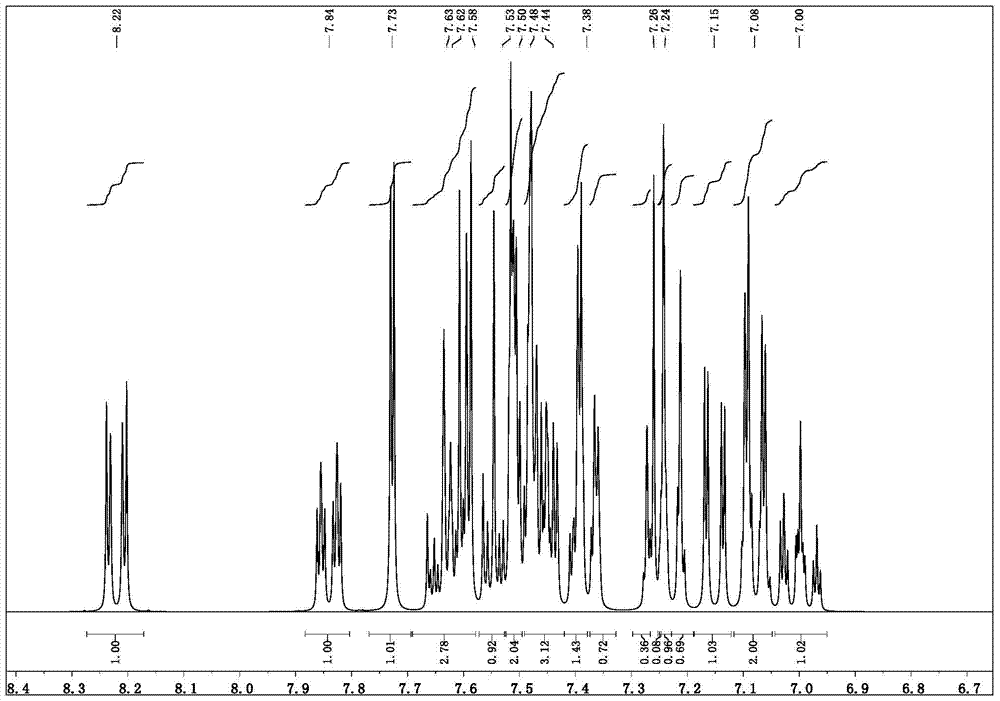
[0044] Synthesis of parent 5,5'-dibromo-1,1'-diphenyl-1H,1'H-2,2'-bibenzo[d]imidazole
[0045] The synthesis steps are the same as the previous 1 and 2, except that in the first step, 1H-benzo[d]imidazole is changed to 5-bromo-1H-benzo[d]imidazole, p-bromoiodobenzene is changed to iodobenzene, other reagents In the second step, use the monobromide synthesized in the first step here, other reagents and reaction conditions remain unchanged, and obtain the target parent 5,5'-dibromo-1,1'-diphenyl Base-1H,1'H-2,2'-bibenzo[d]imidazole. The reaction pathway is as follows:
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com