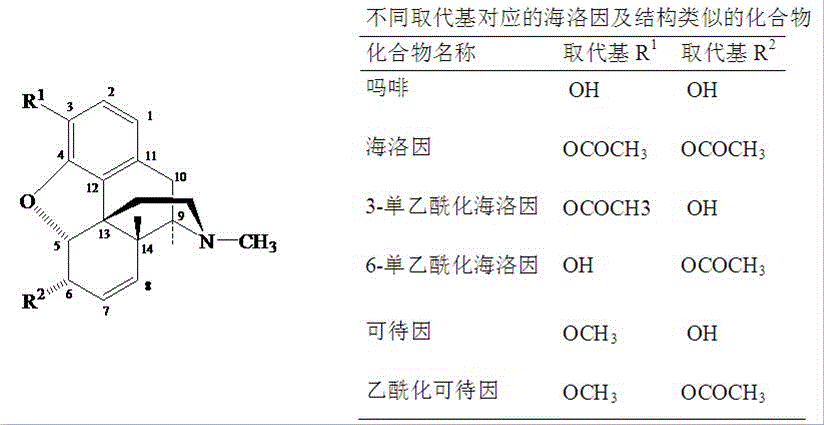
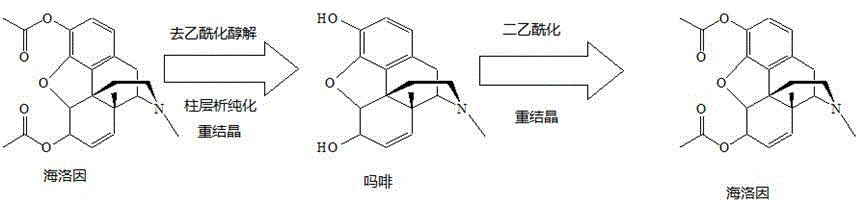
Preparation process of standard products of morphine base and heroin base
A technology for heroin and standard products, which is applied in the field of preparation of heroin base and morphine base standard products, can solve the problems of a large number of high-purity reagents and high-priced equipment investment, and achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Preparation of standard substances of heroin base and morphine base
[0026] Morphine preparation:
[0027] Acid hydrolysis: 50 grams of crude heroin for experiment, with 3mol L -1 300ml of hydrochloric acid for deacetylation reaction. After filtering the resulting solution, heat at 80°C for 60 minutes. After cooling, adjust the pH to neutral with solid sodium hydroxide (precipitation occurs), and then use saturated aqueous sodium carbonate to adjust the pH to 9 (until sodium carbonate is added dropwise). Precipitation occurs again), and the precipitate was left for 5 hours, suction filtered, washed with water, and the precipitate was dried by infrared to obtain 35 g of crude morphine, with a conversion rate of 70%.
[0028] Refining of morphine: The crude morphine was ultrasonically washed with 10 times the amount of diethyl ether, filtered 3 times, dried, purified by silica gel chromatography, and recrystallized from methanol 4 times. Get 17.6 grams of m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com