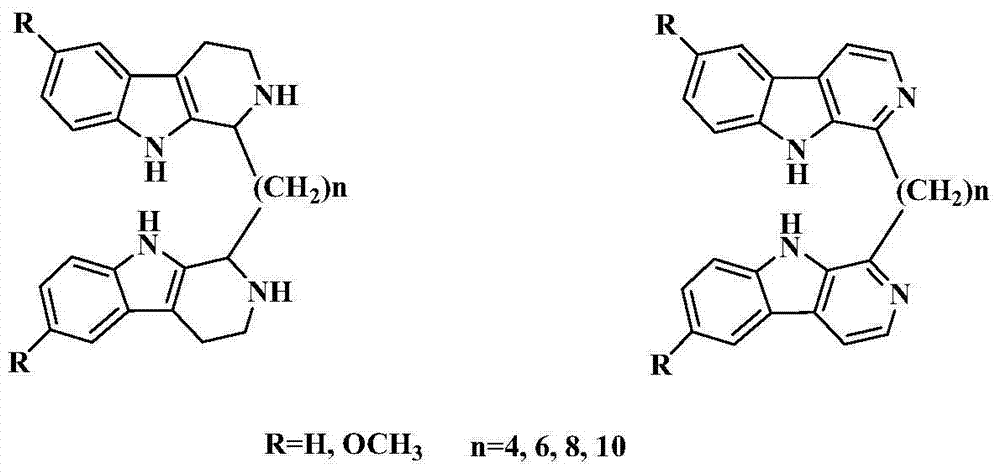
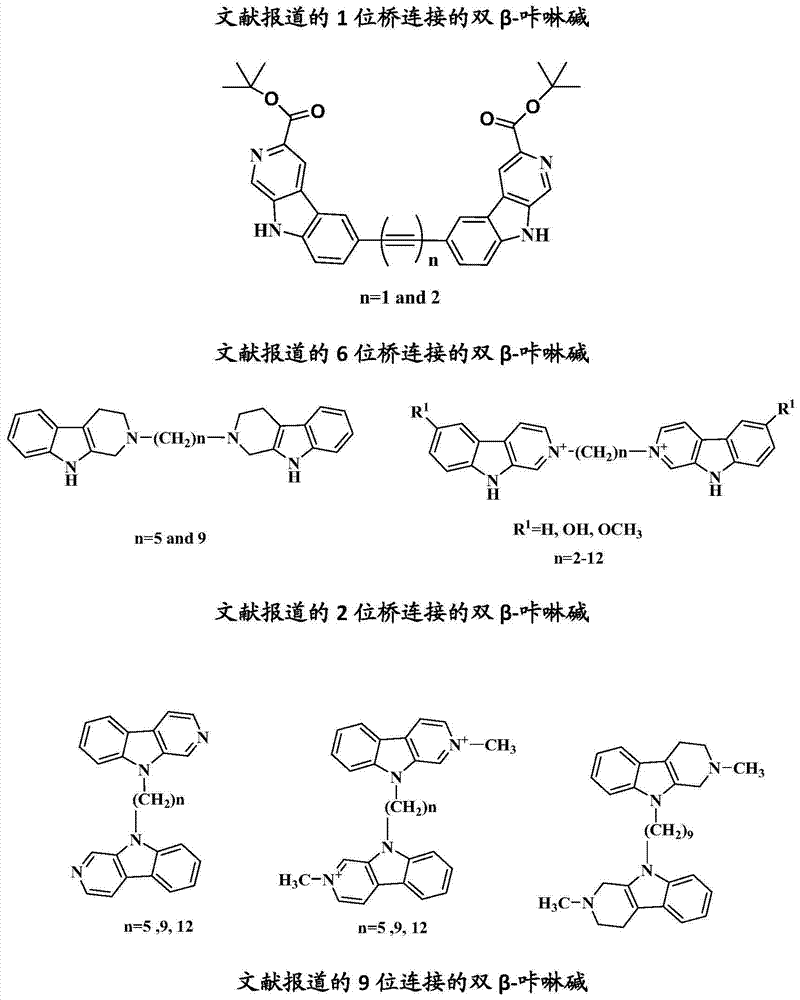
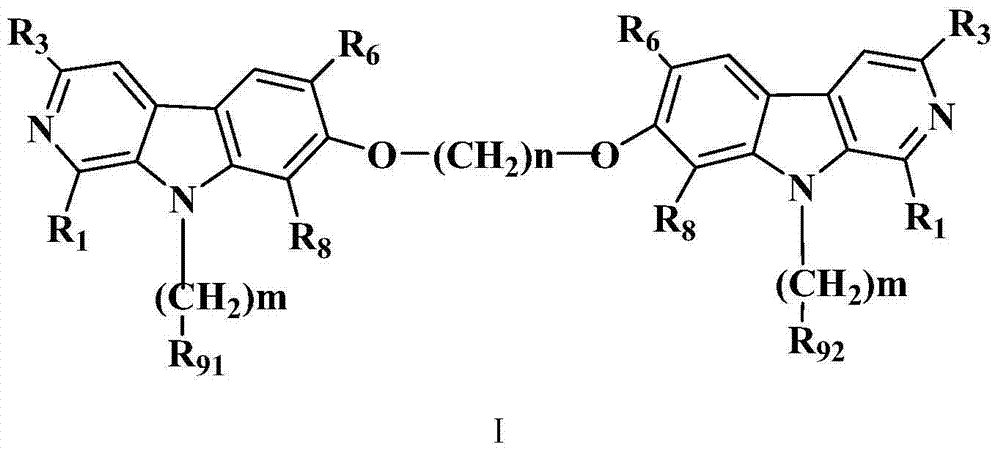
7 site-connected bis(beta-carboline alkaloid) compound and its preparation method, pharmaceutical composition and use
A compound, carboline base technology, applied in the preparation of anti-tumor drugs, the field of 7-position double β-carboline base compounds, can solve the problem of not disclosing the anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0178]
[0179] 1,9-Dimethyl-β-carboline-7-ol:
[0180] Synthesis of 1,9-Dimethyl-β-carboline-7-ol:
[0181] (a) Synthesis of 7‐methoxy‐1,9‐dimethyl‐β‐carboline:
[0182] Add dehydrohaeline (2.12g, 10mmol) and DMF (50ml) into a 100ml eggplant-shaped bottle, stir well, add NaH (0.8g, 20mmol) and stir for 10min, then add iodomethane (1.73ml, 15mmol) and stir at room temperature , TLC tracking detection. After the reaction was completed, the reaction solution was poured into 200 ml of ice water, and extracted three times with ethyl acetate. The organic phases were combined, washed with water and saturated brine. Add saturated hydrogen chloride-ethanol solution to the organic phase to pH = 2-3, distill under reduced pressure, recrystallize from acetone, and filter to obtain a yellow solid. The above solid was dissolved in 100ml of water, with NaHCO 3 Adjust pH=8‐9, extract with ethyl acetate three times, combine organic phases, wash with water and saturated brine. The org...
preparation example 2
[0186]
[0187] Synthesis of 9‐ethyl‐1‐methyl‐β‐carboline‐7‐ol:
[0188] (a) Synthesis of 9‐ethyl‐7‐methoxy‐1‐methyl‐β‐carboline:
[0189] Add dehydrohaeline (2.12g, 10mmol) and DMF (50ml) into a 100ml eggplant-shaped bottle, stir well, add NaH (0.8g, 20mmol) and stir for 10min, then add iodoethane (1.6ml, 15mmol) at room temperature Stirring, TLC follow-up detection. After the reaction was completed, the reaction solution was poured into 200 ml of ice water, and extracted three times with ethyl acetate. The organic phases were combined, washed with water and saturated brine. Add saturated hydrogen chloride-ethanol solution to the organic phase to pH = 2-3, distill under reduced pressure, crystallize acetone, and filter to obtain a yellow solid. The above solid was dissolved in 100ml of water, with NaHCO 3 Adjust the pH to 8‐9, extract 3 times with ethyl acetate, combine the organic phases, wash with water, and wash with saturated brine. The organic phase was dehydrate...
preparation example 3
[0193]
[0194] Synthesis of 9-n-butyl-1-methyl-β-carboline-7-ol:
[0195] (a) Synthesis of 9-n-butyl-7-methoxy-1-methyl-β-carboline:
[0196] Add dehydrobamaline (2.12g, 10mmol) and DMF (50ml) into a 100ml eggplant-shaped bottle, stir well, add NaH (0.8g, 20mmol) and stir for 10min, then add iodo-n-butane (1.38ml, 15mmol ) Stirring at room temperature, followed by TLC detection. After the reaction was completed, the reaction solution was poured into 200 ml of ice water, and extracted three times with ethyl acetate. The organic phases were combined, washed with water and saturated brine. Add saturated hydrogen chloride-ethanol solution to the organic phase to pH = 2-3, distill under reduced pressure, crystallize acetone, and filter to obtain a yellow solid. The above solid was dissolved in 100ml of water, with NaHCO 3 Adjust the pH to 8‐9, extract 3 times with ethyl acetate, combine the organic phases, wash with water, and wash with saturated brine. The organic phase w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com