In vitro pancreatic differentiation of pluripotent mammalian cells
A pluripotent cell and cell technology, applied in animal cells, vertebrate cells, artificial cell constructs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0201] experiment
[0202] hESC and hIPSC culture conditions.
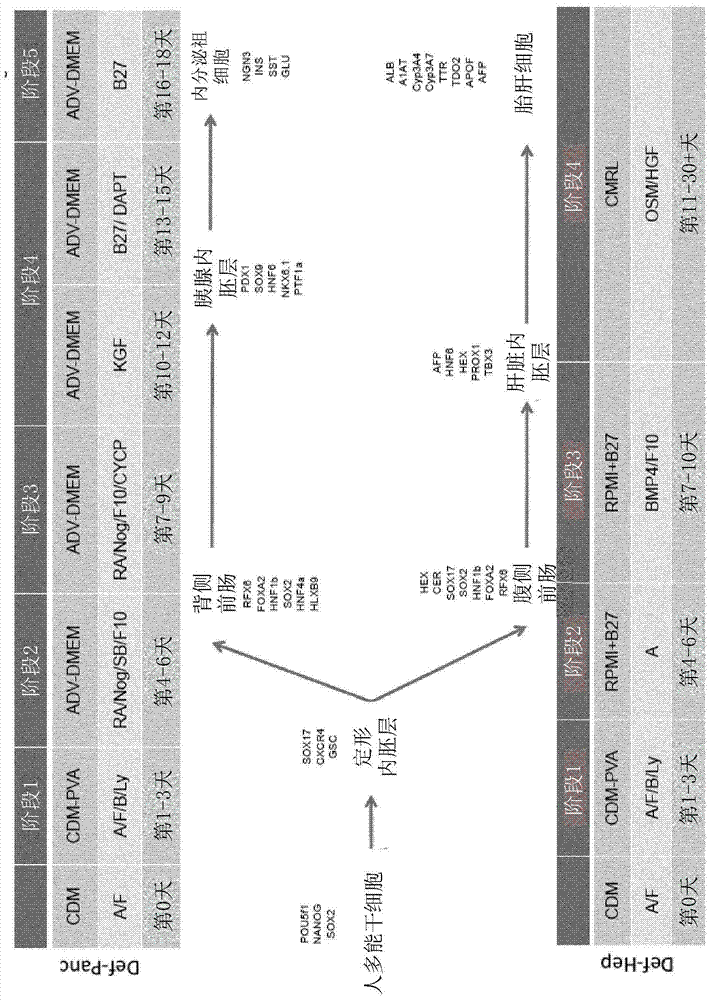
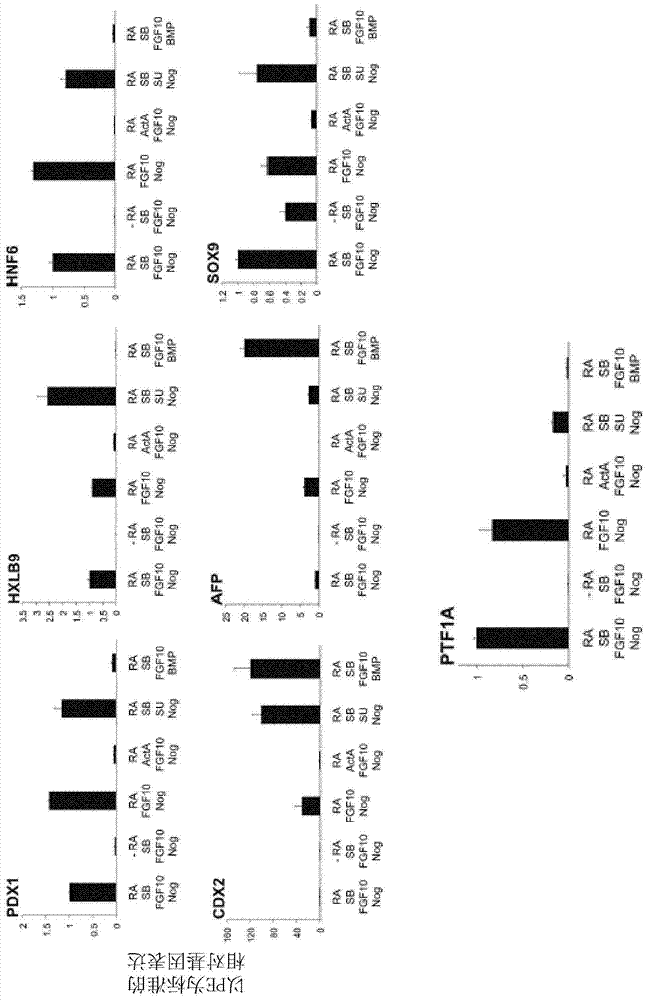
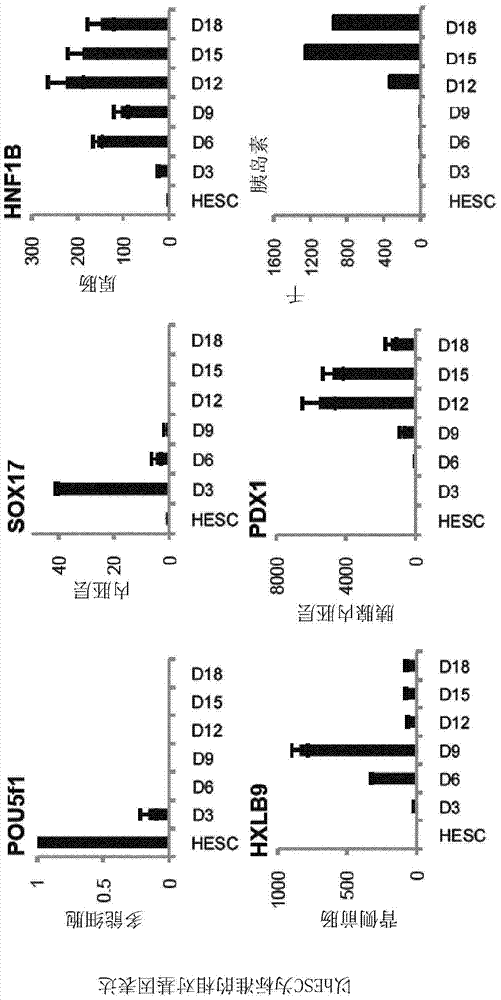
[0203] hESCs (H9 purchased from WiCell) and hIPSCs (BBHX8, A1ATD-1, JRO1D) (Rashid S et al. (2010) J. Clin. Invest. 120: 3127-3136) were grown in defined media (Brons et al. ( 2007) Nature 448:191-195). Cells were passaged weekly with collagenase IV and maintained in chemically defined medium (CDM) supplemented with activin A (10 ng / ml) and FGF2 (12 ng / ml) as previously described (Brons et al. )middle. differentiated as figure 1 in the manner described. Medium was changed daily throughout the differentiation process. After the DE stage (Stage 1), the cells were treated with SB-431542 (10 μM; Tocris), FGF10 (50 ng / ml; AutogenBioclear), all-trans retinoic acid (RA, 2 μM; Sigma) and Tau Protein (50ng / ml; R&D Systems) was cultured in advanced DMEM (Invitrogen) for 3 days. In stage 3, the cells were incubated with high-grade DMEM+human FGF10 (50ng / ml; AutogenBioclear), all-trans retinoic acid (RA, 2μM; Sigma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com