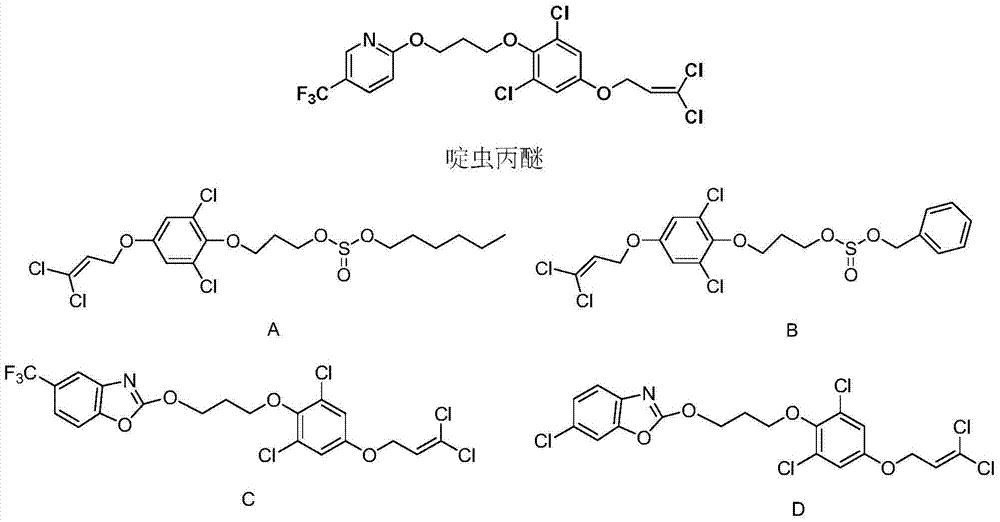
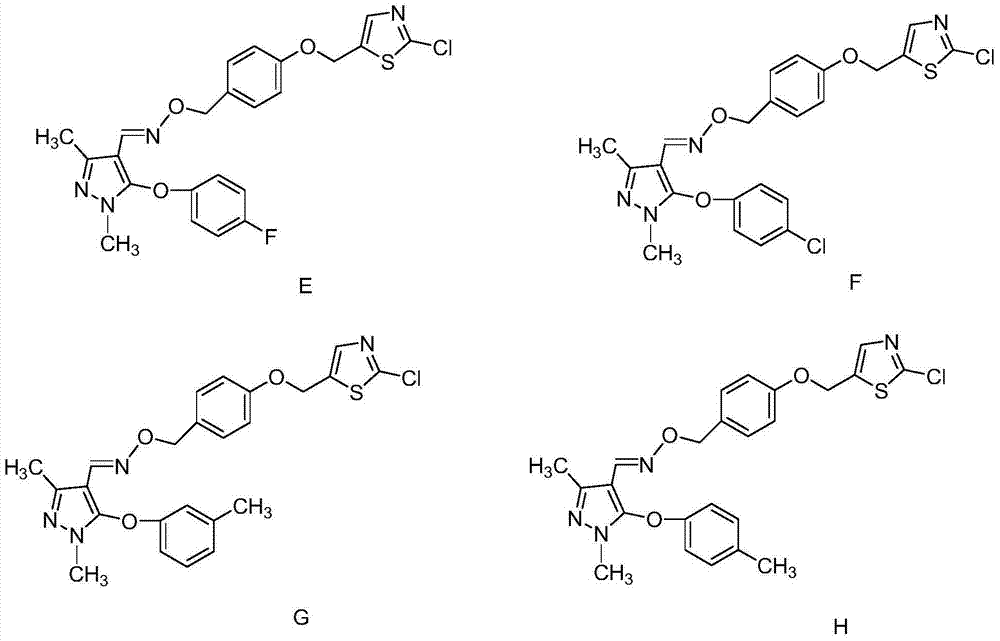
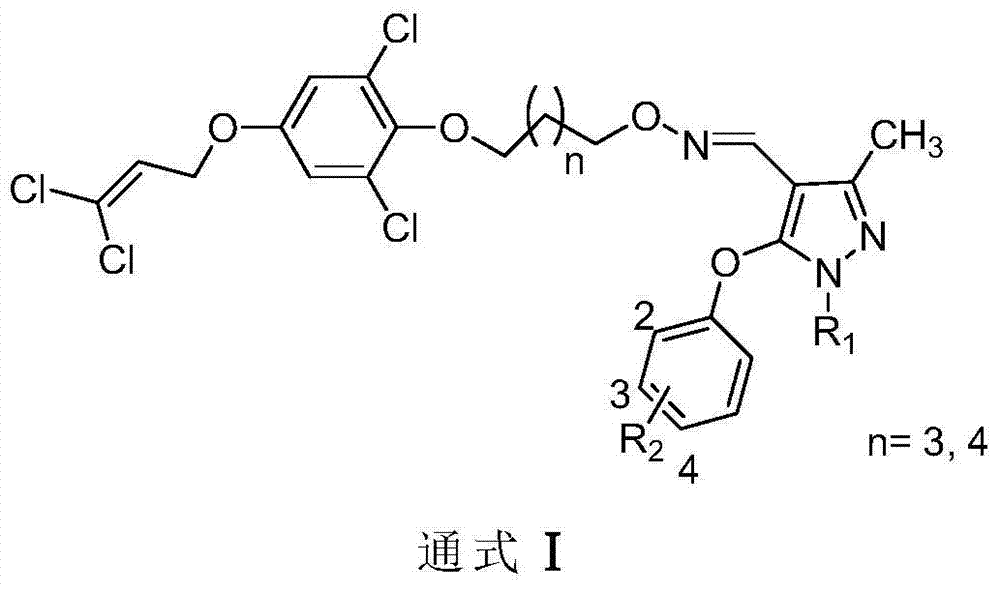
1, 1-dichloropropene ether compound containing n-substituent-3-methylpyrazole oxime unit structure, preparation method and application thereof
A technology of dichloropropene ethers and methylpyrazole oxime, which is applied in the field of pesticides to achieve excellent control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of compound Ia (n=3 in general formula II, R in III 1 for -CH 3 , R 2 for 4-F)
[0036]
[0037] In a 100 mL flask, 3 mmol of compound III and 20 mL of N,N-dimethylformamide were added, and then 4 mmol of compound II and 9 mmol of potassium carbonate were added thereto. Slowly heated to 80°C for 8 hours. Then the reaction solution was cooled, filtered with suction, and the solid was removed by filtration, the mother liquor was concentrated under reduced pressure, and purified by column chromatography to obtain the target compound Ia as a yellow oil. 1 H NMR (400MHz, CDCl 3 )7.75(s,1H,CH=N),6.98-7.02(m,2H,ArH),6.83-6.88(m,4H,ArH),6.12(t,J=6.0Hz,1H, CH CH 2 ), 4.58 (d, J=6.0Hz, 2H, CHC H 2 O),3.92-4.01(m,4H,CH 2 OAr and C H 2 O-N=CH),3.61(s,3H,N-CH 3 ),2.37(s,3H,CH 3 ),1.80-1.84(m,2H,CH 2 ),1.53-1.67(m,4H,2×CH 2 ).
Embodiment 2
[0039] Synthesis of compound Ib (n=3 in general formula II, R in III 1 for-Ph,R 2 for 4-F)
[0040]In a 100 mL flask, 4 mmol of compound III and 25 mL of butanone were added, and then 4 mmol of compound II and 6 mmol of potassium tert-butoxide were added thereto. Heat to reflux for 11 hours. Then the reaction liquid was cooled, filtered with suction, and the solid was removed by filtration, the mother liquor was concentrated under reduced pressure, and purified by column chromatography to obtain the target compound Ib as a yellow oil. 1 H NMR (400MHz, CDCl 3 )7.82(s, 1H, CH=N), 7.61(d, J=8.0Hz, 2H, ArH), 7.27-7.41(m, 3H, ArH), 6.90-7.00(m, 4H, ArH), 6.86( s,2H,ArH),6.13(t,J=6.4Hz,1H, CH CH 2 ), 4.60 (d, J=6.4Hz, 2H, CHC H 2 O),3.95-4.07(m,4H,CH 2 OAr and C H 2 O-N=CH),2.50(s,3H,CH 3 ),1.84-1.88(m,2H,CH 2 ),1.57-1.72(m,4H,2×CH 2 ).
Embodiment 3
[0042] Synthesis of compound Ic (n=4 in general formula II, R in III 1 for -CH 3 , R 2 for 4-I)
[0043] In a 100mL flask, add 2mmol of compound III and 25mL of THF, then add 4mmol of sodium bicarbonate to it at room temperature, stir for a while, add 2.5mmol of compound II to it, and heat to reflux for 9 hours. Then the reaction solution was cooled, filtered with suction, and the solid was removed by filtration, the mother liquor was concentrated under reduced pressure, and purified by column chromatography to obtain the target compound Ic as a yellow oil. 1 H NMR (400MHz, CDCl 3 )7.77(s,1H,CH=N),7.61(d,J=8.8Hz,2H,ArH),6.85(s,2H,ArH),6.70(d,J=9.2Hz,2H,ArH),6.13 (t,J=6.4Hz,1H, CH CH 2 ), 4.60 (d, J=6.4Hz, 2H, CHC H 2 O),3.94-3.99(m,4H,CH 2 OAr and C H 2 O-N=CH),3.62(s,3H,N-CH 3 ),2.38(s,3H,CH 3 ),1.80-1.87(m,2H,CH 2 ),1.50-1.61(m,4H,2×CH 2 ), 1.38-1.44 (m,2H,CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com