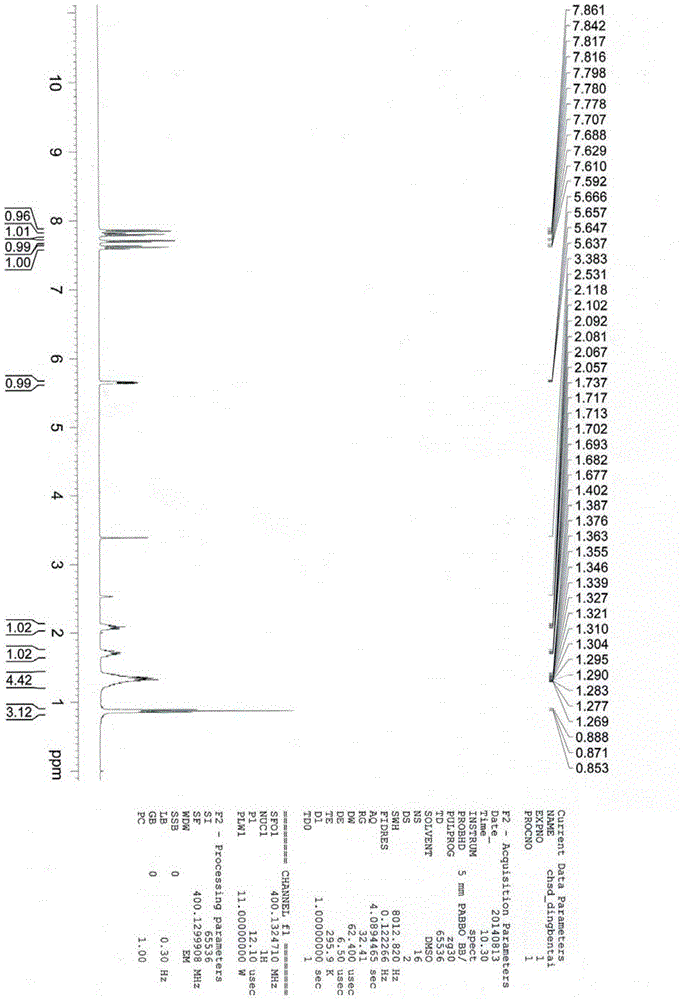
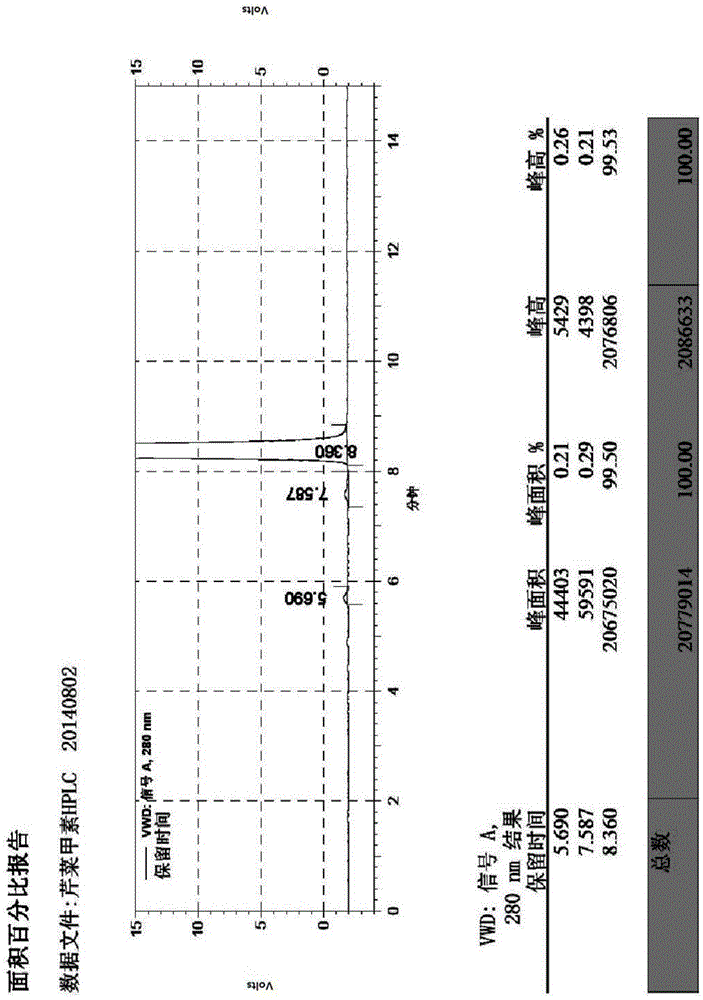
Synthetic method of butylphthalide
A technology of apigenin A and tetrahydrofuran, which is applied in the field of chemical industry and can solve the problems such as complicated operation, many reaction steps, and difficulty in obtaining raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] (1), Preparation of 3-butynylphthalide
[0031] Under nitrogen protection, add 100ml of tetrahydrofuran to a four-necked 500ml flask equipped with mechanical stirring, a thermometer and a drying tube, add 0.12mol of butyne sodium in 100ml of tetrahydrofuran solution (0.12mol of butyne sodium is dissolved in 100ml of tetrahydrofuran), cool down To -40°C, add dropwise 21.3 grams of 0.1mol 3-bromophthalide in 50ml tetrahydrofuran solution under stirring, control the temperature at -40--35°C and add dropwise, after the addition is complete, continue stirring for 1 hour; then use saturated saline The reaction liquid was washed, and the organic layer was separated and dried with sodium sulfate; after the desiccant was removed by filtration, the solvent tetrahydrofuran was distilled off under reduced pressure. 17.8 g of 3-butynylphthalide oil was obtained, HPLC92.7%, and purified by silica gel column chromatography to obtain 14.7 g of product, HPLC99.8%. The yield was 79%.
...
Embodiment 2
[0035] (1), Preparation of 3-butynylphthalide
[0036] Under nitrogen protection, add 100ml of tetrahydrofuran to a four-necked 500ml flask equipped with mechanical stirring, a thermometer and a drying tube, add 0.12mol of butyne sodium in 100ml of tetrahydrofuran solution (0.12mol of butyne sodium is dissolved in 100ml of tetrahydrofuran), cool down To -40°C, add dropwise 21.3 grams of 0.1mol 3-bromophthalide in 50ml tetrahydrofuran solution under stirring, control the temperature at -40--35°C and add dropwise, after the addition is complete, continue stirring for 1 hour; then use saturated saline The reaction liquid was washed, and the organic layer was separated and dried with sodium sulfate; after the desiccant was removed by filtration, the solvent tetrahydrofuran was distilled off under reduced pressure. 17.0 g of 3-butynylphthalide oil was obtained, HPLC92.3%, and purified by silica gel column chromatography to obtain 14.1 g of product, HPLC99.8%. The yield was 75.8%. ...
Embodiment 3
[0040] (1), Preparation of 3-butynylphthalide
[0041] Under nitrogen protection, equipped with mechanical stirring, a thermometer and a four-necked 1000ml flask with a drying tube, add 200ml tetrahydrofuran, add 200ml tetrahydrofuran solution of 0.24mol sodium butyne (dissolve 0.24mol sodium butyne in 200ml tetrahydrofuran), cool down To -40°C, add dropwise 42.5g of 0.2mol 3-bromophthalide in 100ml tetrahydrofuran solution under stirring, control the temperature at -40--35°C and add dropwise, after the dropwise addition is complete, continue stirring for 1 hour. Then, the reaction solution was washed with saturated brine, and the organic layer was separated and dried with sodium sulfate. After removing the desiccant by filtration, the solvent tetrahydrofuran was distilled off under reduced pressure. 35.5 g of 3-butynylphthalide oil was obtained, HPLC92.1%, and purified by silica gel column chromatography to obtain 29.2 g of product, HPLC99.83%. The yield was 78.5%.
[0042...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com