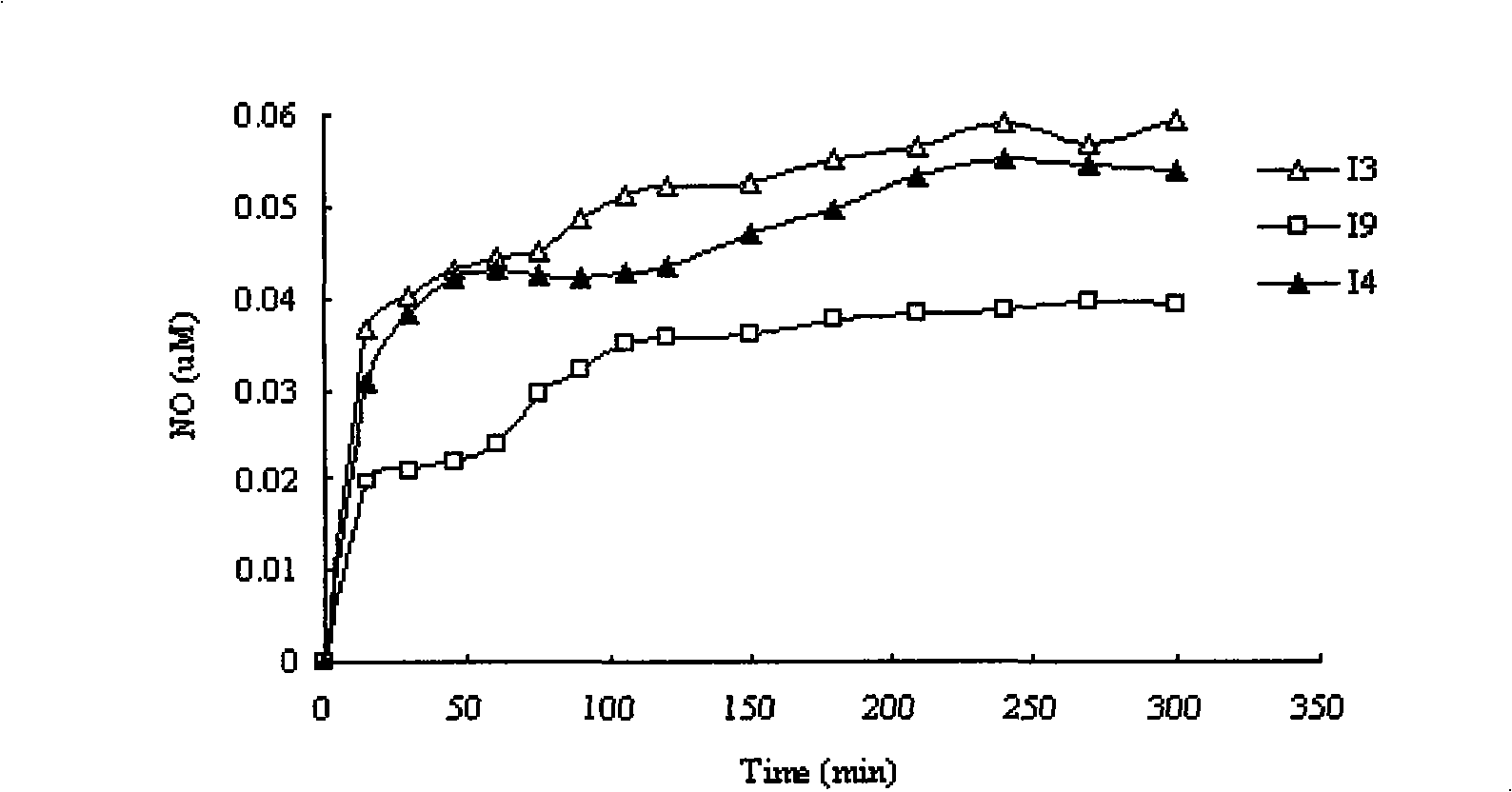
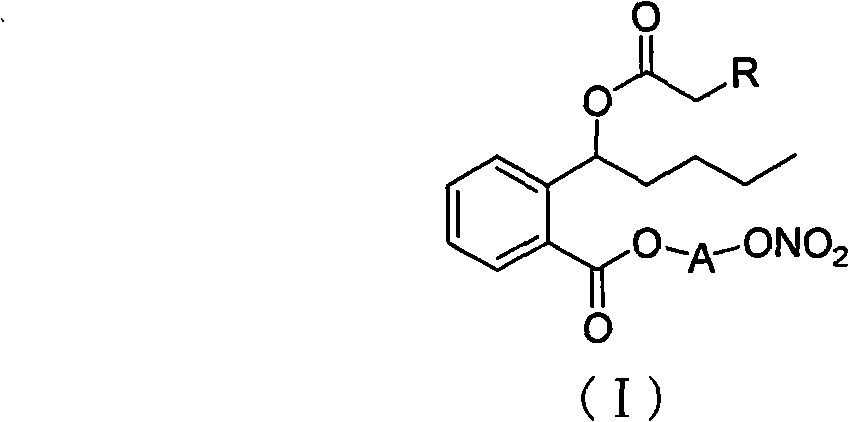
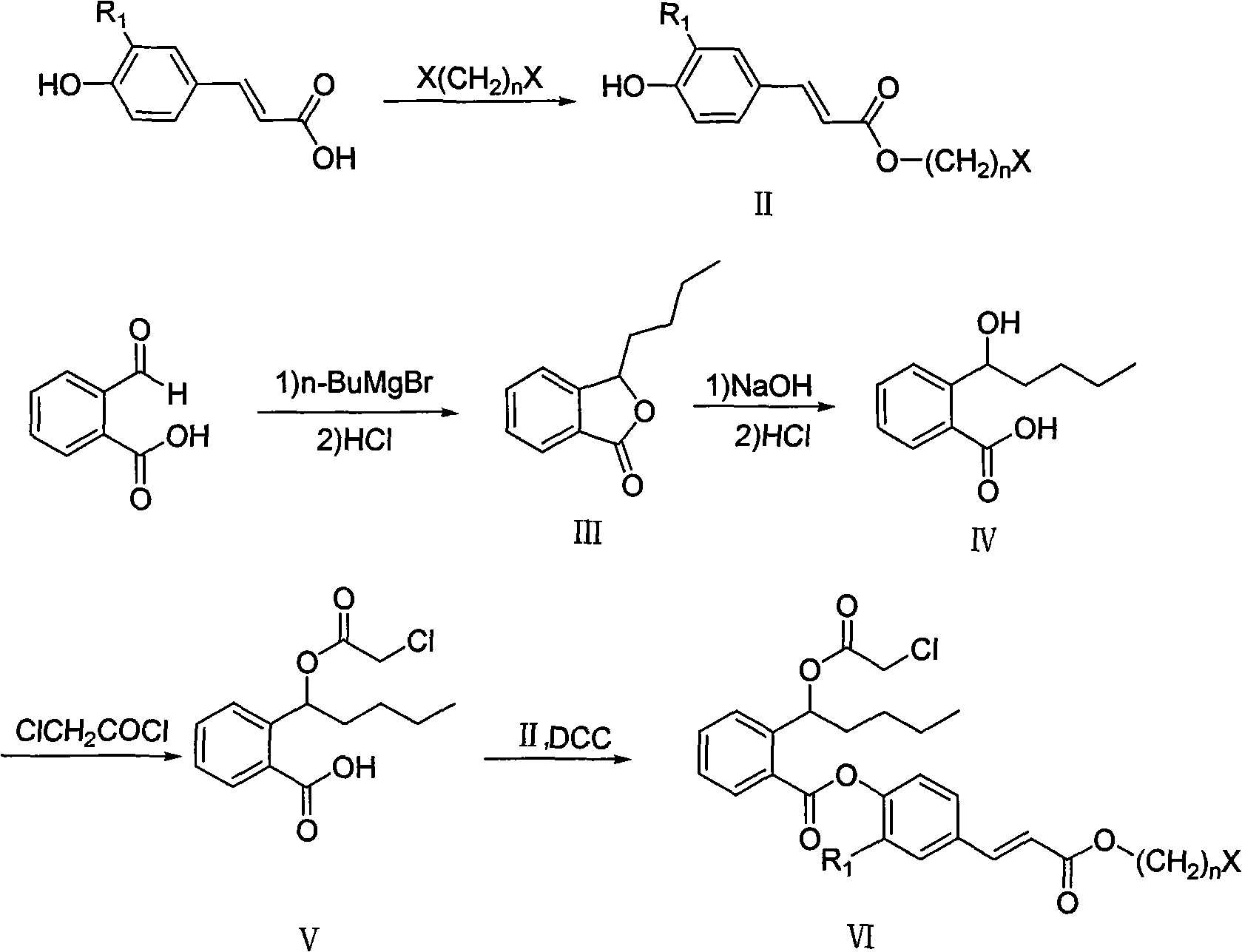
Nitric oxide donor type 3-butylphthalide derivates, method for preparing same and pharmaceutical use
A technology of butylphthalide and derivatives, applied in the field of antithrombotic and anti-cerebral ischemia drugs, can solve problems to be strengthened, and achieve the effect of enhancing anti-cerebral ischemia, antithrombotic and obvious effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Preparation of 3-butylphthalide (III)
[0104] Add 1.5g (0.01mol) o-formylbenzoic acid and 30mL Et 2 O, N 2 Protect, stir to dissolve. At -5°C, add 30 mL of diethyl ether suspension containing 3.6 g (22 mmol) n-BuMgBr dropwise. 4 The reaction was quenched with Cl solution, and 15 mL of 10% hydrochloric acid was added, and stirred at room temperature for 2 h. Separate the organic phase, extract the aqueous layer with 3×30mL ether, combine the organic layers, Na 2 SO 4 After drying, concentration, and column chromatography [petroleum ether: ethyl acetate (v:v) = 15:1], 1.24 g of light yellow oil was obtained, with a yield of 75%. ESIMS (m / z): 213 [M+Na]+; 1 HNMR (CDCl 3 )δ: 0.93(t, 3H, CH 3 , J=6.2Hz), 1.37-1.45(m, 4H, 2×CH 2 ), 1.86-1.89 (m, 2H, CH 2 ), 5.49 (q, 1H, CH, J=12.3Hz), 7.37-7.39 (m, 1H, ArH, H-4), 7.56-7.5 (m, 2H, ArH), 8.05 (d, 1H, ArH, H-7, J=6.5Hz).
Embodiment 2
[0106] Preparation of 2-(1-hydroxy-n-pentyl)benzoic acid (IV)
[0107] Dissolve 1.24g (6.5mmol) III in a 50mL single-neck flask containing 10mL of methanol, add 10mL of 2M NaOH solution, stir at 50°C for 1h, evaporate methanol under reduced pressure, add 10mL of distilled water to dilute, cool to -5°C, and vigorously Acidify with 5% hydrochloric acid to pH 2-3 under stirring, extract with ether, Na 2 SO 4 dry. Ether was vacuum-dried under 0°C to obtain 1.32 g of white solid, yield 90%. The product was unstable and was directly put into the next reaction without purification.
Embodiment 3
[0109] Preparation of 2-(1-chloroacetoxy-n-pentyl)benzoic acid (V)
[0110] Put 1.04g (5mmol) of IV, 2mL of triethylamine, 0.5g of DMAP and 20mL of dichloromethane into a 50mL two-neck flask, stir and dissolve at -10°C-0°C, add dropwise 1.2mL (15mmol) of chloroacetyl chloride, Insulated and stirred for 5h. Add 10 mL of water, stir at room temperature for 0.5 h, separate the organic layer, Na 2 SO 4 Dry, filter, and concentrate to obtain a waxy solid, which is recrystallized from n-hexane to obtain 0.92 g of white needle-shaped crystals, with a yield of 65%. m.p.67-68°C; ESIMS: m / z 283[ 35 ClM-H + ], 285[ 37 ClM-H + ]; 1 HNMR (300MHz, CDCl 3 , δppm): 0.91 (t, 3H, CH 3 , J=6.9Hz), 1.32-1.41(m, 4H, 2×CH 2 ), 1.86-1.91 (m, 2H, CH 2 ), 4.11 (s, 2H, CH 2 CO), 6.76-6.79(m, 1H, CH), 7.36-7.42(m, 1H, ArH, H-4), 7.56-7.62(m, 2H), 8.08(d, 1H, ArH, H-7, J=8.1Hz), 10.89 (s, br, 1H, COOH); IR (KBr, v): 1412, 1691, 1734, 2958, 3450cm -1 ;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com