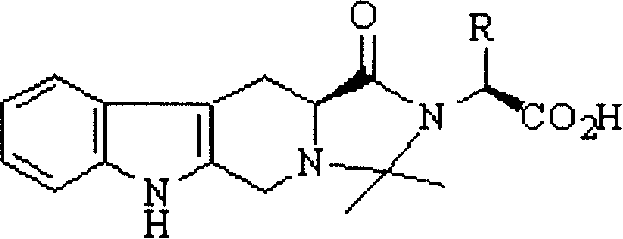
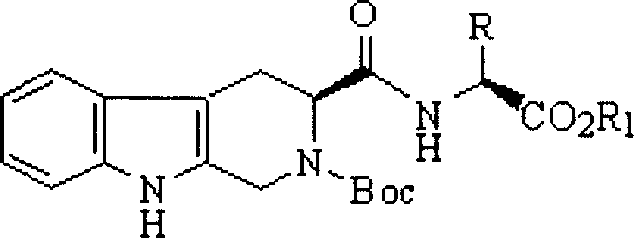
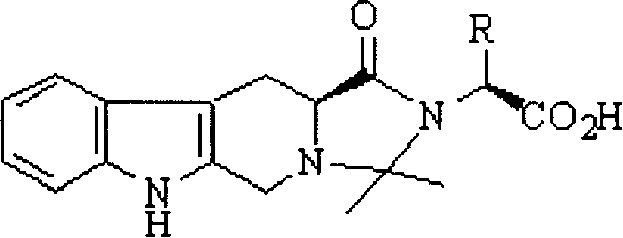
Anti-thrombus N-butyl-2,2-dimethyl-4-oxy-tetrahydroimidazopyridoindole and its synthesis and application
A technology of tetrahydroimidazole and dimethyl, which is used in medical preparations containing active ingredients, blood diseases, drug combinations, etc., can solve the problems of low bioavailability, affecting treatment and use effects, and poor solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 1-(1'-Carboxy-2'-methyl)butyl-2,2-dimethyl-4-oxo-tetrahydroimidazo[1',2':1,5]pyrido Preparation of [3,4-b]indole (2a)
[0051] 1) Preparation of S-carboline carboxylic acid
[0052] 400ml of water was placed in a 500ml round bottom flask, and 0.2ml of concentrated sulfuric acid was slowly added. 5.0 g (24.5 mmol) of L-tryptophan was added to the obtained dilute aqueous sulfuric acid solution and sonicated until the L-tryptophan was completely dissolved. To the resulting solution was added 10 ml of 35% formaldehyde solution. The reaction mixture was stirred at room temperature for 6 hours, and the disappearance of L-tryptophan was monitored by thin layer chromatography to terminate the reaction. Concentrated ammonia water was slowly added dropwise to the reaction solution, the pH of the reaction mixture was adjusted to 6, and it was allowed to stand for half an hour. The resulting precipitate filtered out under reduced pressure was washed with water, the co...
Embodiment 2
[0061] Example 2 N-(1'-Carboxy-2'-methyl)propyl-2,2-dimethyl-4-oxo-tetrahydroimidazo[1',2':1,5]pyrido Preparation of [3,4-b]indole (2b)
[0062] 1) Preparation of N-Boc-S-carbolineyl-L-valine methyl ester
[0063] According to the operation of preparing N-Boc-S-carbolineyl-L-isoleucine methyl ester in Example 1, 2.65 g (97.5%) target was obtained from 2.00 g (6.33 mmol) L-valine methyl ester hydrochloride The compound is a colorless solid. Mp 138-140°C; ESI / MS 430 [M+H] + .IR(KBr): 3443, 3202, 3001, 2951, 2845, 1729, 1648, 1602, 1450, 1392, 1370, 1067, 902cm -1 ; 1 H NMR (BHSC-500, DMSO-d 6 ): δ=10.043(s, 1H), 7.962(s, 1H), 7.295(t, J=7.4Hz, 1H), 7.211(t, J=7.7Hz, 1H), 7.004(d, J=7.7Hz) , 1H), 6.892 (d, J=7.4Hz, 1H), 4.840 (t, J=5.4Hz, 1H), 4.423 (d, J=5.4Hz, 1H), 4.225 (dd, J=10.2Hz, J =4.5Hz, 1H), 4.037(dd, J=10.2Hz, J=3.7Hz, 1H), 3.626(s, 3H), 3.103(m, J=5.4Hz, 1H), 2.951(d, J=6.7 Hz, 2H), 1.474 (s, 9H), 1.053 (d, J=5.4Hz, 6H). Elemental Analysis C 23 H 31 N 3 O ...
Embodiment 3
[0068] Example 3 1-(1'-Carboxy-3'-methyl)butyl-2,2-dimethyl-4-oxo-tetrahydroimidazo[1',2':1,5]pyrido Preparation of [3,4-b]indole (2c)
[0069] 1) Preparation of N-Boc-S-carbolineyl-L-leucine methyl ester
[0070] According to the operation of preparing N-Boc-S-carbolineyl-L-isoleucine methyl ester in Example 1, 2.78 g (99.2%) target was obtained from 1.20 g (6.61 mmol) L-leucine methyl ester hydrochloride The compound is a colorless solid. IR(KBr): 3443, 3205, 3002, 2954, 2845, 1724, 1642, 1605, 1455, 1392, 1370, 1065, 903cm -1 ;ESI + -MS(m / e)444[M+H] + ; 1H NMR (BHSC-500, DMSO-d 6 ): δ=10.043(s, 1H), 7.962(s, 1H), 7.291(t, J=7.4Hz, 1H), 7.212(t, J=7.4Hz, 1H), 7.004(d, J=7.4Hz) , 1H), 6.891 (d, J=7.4Hz, 1H), 4.840 (t, J=5.4Hz, 1H), 4.422 (d, J=5.4Hz, 1H), 4.224 (dd, J=10.2Hz, J =4.5Hz, 1H), 4.036(dd, J=10.2Hz, J=3.7Hz, 1H), 3.625(s, 3H), 2.950(d, J=6.7Hz, 2H), 1.877(d, J=6.0 Hz, 2H), 1.473 (s, 9H), 1.852 (m, J=5.4Hz, 1H), 1.055 (d, J=5.4Hz, 6H). Elemental Analysis C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com