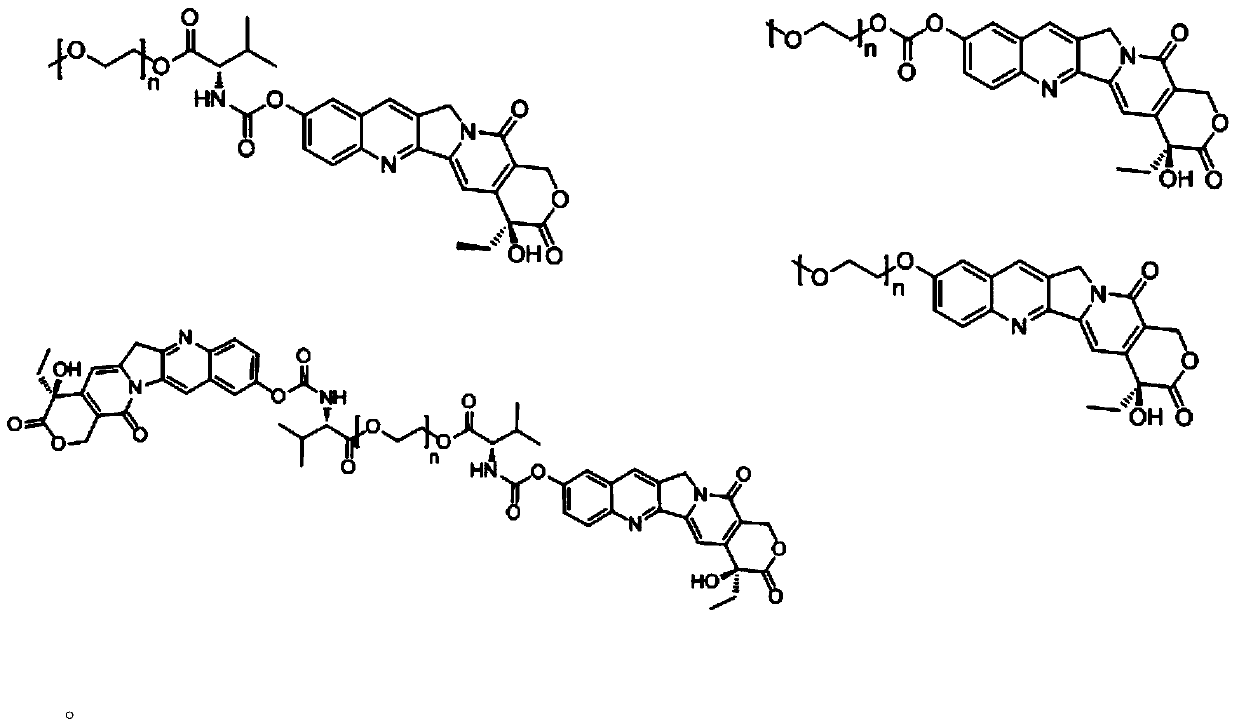
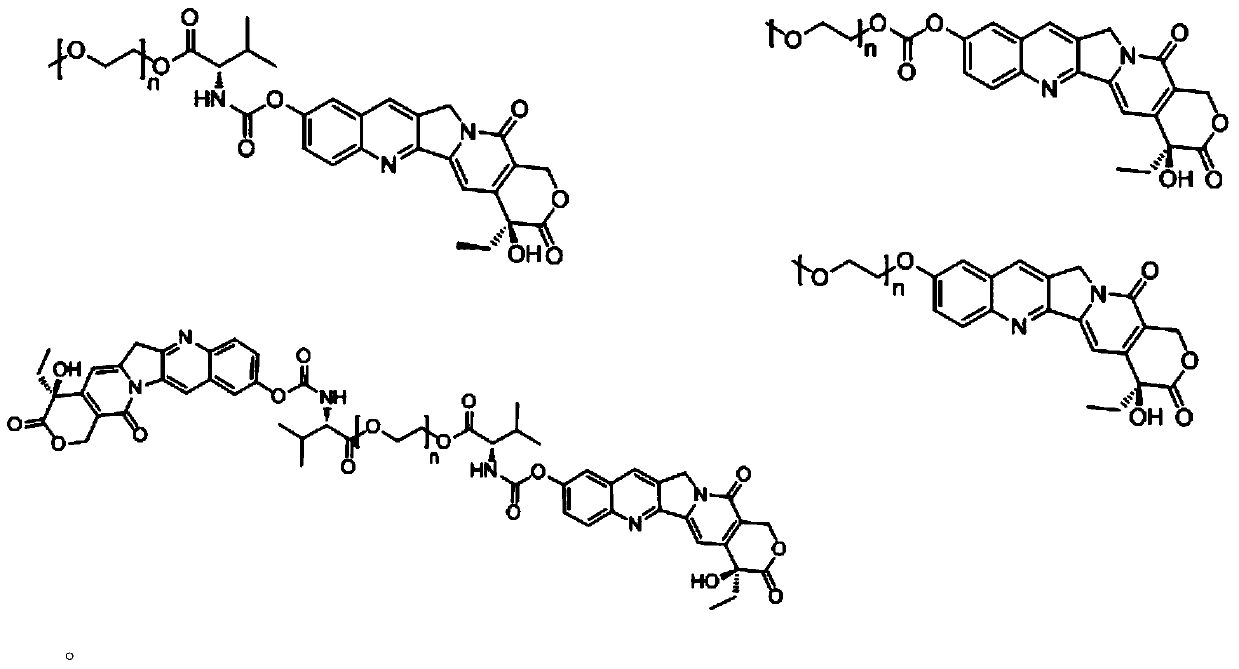
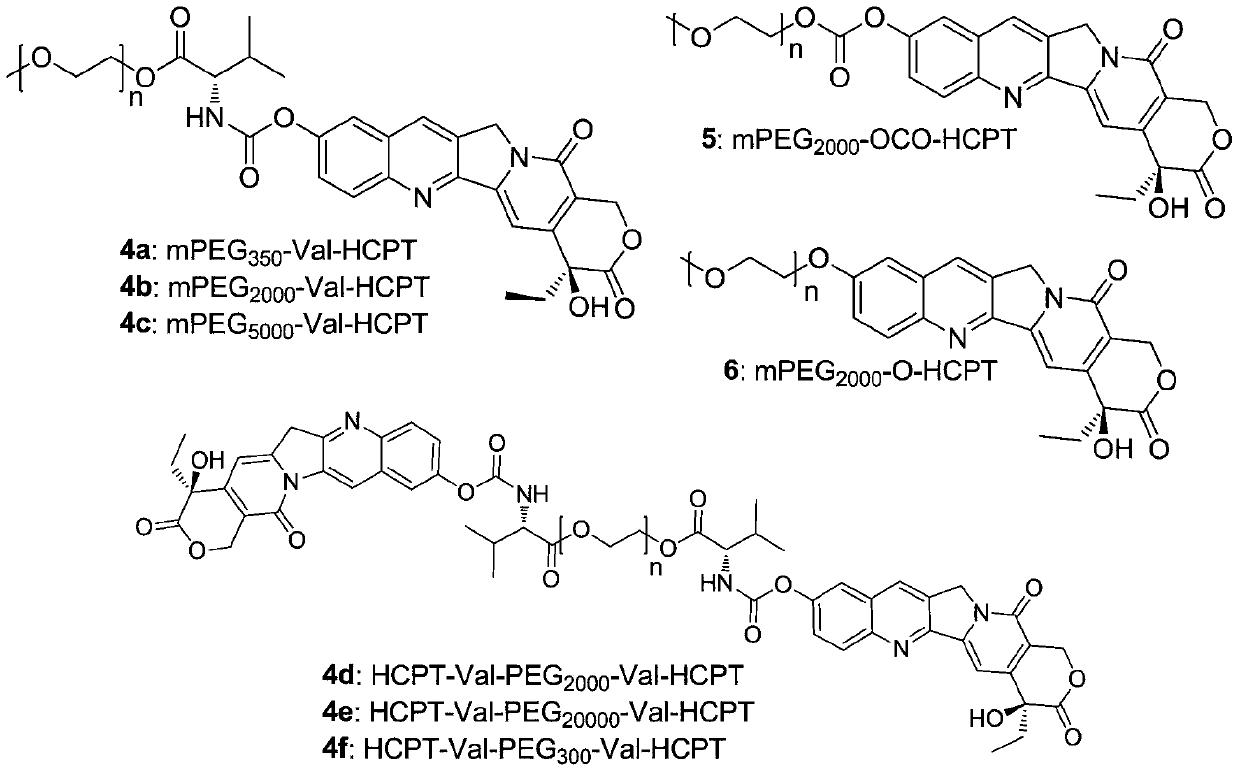
Novel water-soluble polyethylene glycol link-coupled hydroxycamptothecine derivatives and application thereof
A technology of hydroxycamptothecin and polyethylene glycol, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., and can solve unstable metabolism in the body, poor water solubility, and many side effects, etc. problem, to achieve the effect of inhibiting or killing tumor cells, short synthetic route and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Synthesis of 10-(4-nitrophenylcarbonate)-camptothecin (1a)
[0046] Take 3g (8.23mmol) of 10-hydroxycamptothecin and dissolve it in 600mL of anhydrous tetrahydrofuran, add 11.5mL (82.3mmol) of triethylamine under ice bath, and then add 6.64g (32.9mmol) of phenyl p-nitrochloroformate , reacted at room temperature for 1h. The reaction solution was directly spin-dried under reduced pressure, subjected to ethyl acetate, 200-300 mesh silica gel column chromatography, and recrystallized from dichloromethane and n-hexane to obtain 3.59 g of a white solid, which was sent to the next step without further purification, and the crude yield was 82.3%. 1 H NMR (400MHz, DMSO-d 6 )δ (ppm): 8.74 (s, 1H), 8.40 (d, J = 9.2Hz, 2H), 8.30 (d, J = 9.2Hz, 1H), 8.20 (d, J = 2.4Hz, 1H), 7.97 –7.80(m,1H),7.78(d,2H,J=9.2Hz),7.37(s,1H),6.54(s,1H),5.44(s,2H),5.32(s,2H),1.85– 1.92(m,2H),0.89–0.92(m,3H).
[0047] (2) Synthesis of N-tert-butoxycarbonyl valine polyethylene glycol monomethyl eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com