LAMP-and-molecular-beacon-based reaction system and method for detecting HPV16 and HPV18
A reaction system and molecular beacon technology, applied in the field of molecular biology detection, can solve the problems of inability to distinguish positive amplification results from false positive results, inconvenience of clinical detection, etc., and achieve the effect of technical sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Reaction system and method for detecting HPV16 and 18 based on LAMP and molecular beacons
[0047]Select the conserved sequences of HPV16 and HPV18 to design corresponding LAMP primers, including two outer primers (F3, B3) and two inner primers (FIP, BIP), and corresponding molecular beacon probes (MB-16, MB-18 ). Primers were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. and purified by HPLC.
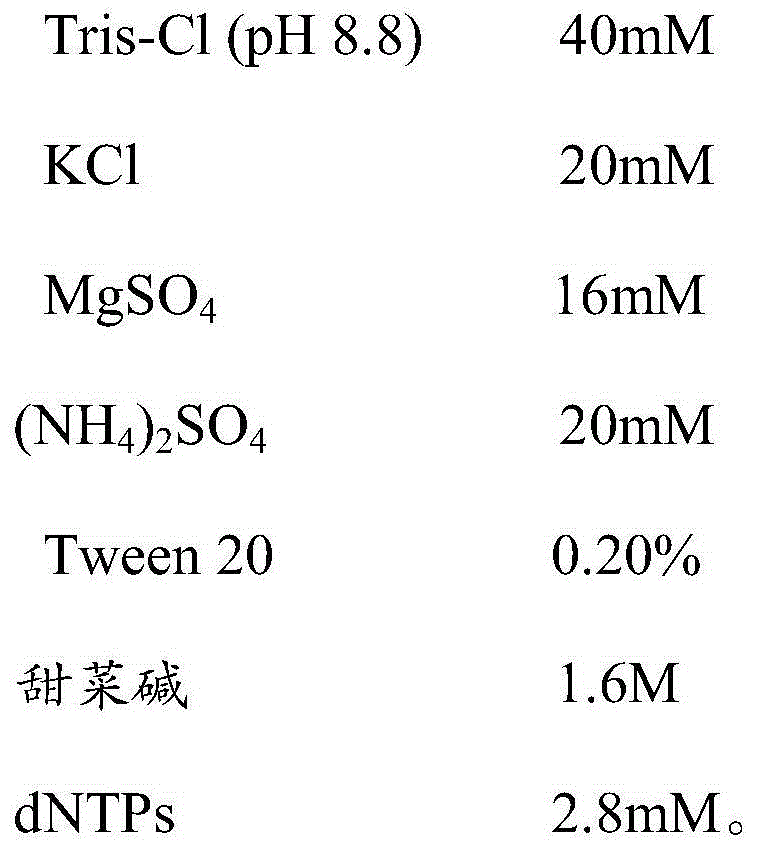
[0048] The reaction system includes a primer set for detecting HPV16 and HPV18, specifically including the following components:
[0049] The reaction system includes the following components:
[0050]
[0051]
[0052] The sequences are as follows:
[0053] CATGGAGATACACCTACATTG (SEQ ID NO.1)
[0054] GTACGGATGTCTACGTGTG (SEQ ID NO. 2)
[0055] CTCTGAGCTGTCATTTAATTGCTCAGAATATATGTTAGATTTGCAACCAG (SEQ ID NO. 3)
[0056] GGACAAGCAGAACCGGACAGCGAAGCGTAGAGTCACAC (SEQ ID NO. 4)
[0057] CGCGAGACGAAATAGATGGTCCAGTCGCG (SEQ ID NO. 5)
[0058] ACAAATGTC...
Embodiment 2
[0071] Embodiment 2 Sensitivity detection of the detection method of embodiment 1
[0072] Use HPV16, type 18 primers F3 (SEQ.ID.NO.1 and SEQ.ID.NO.6), B3 (SEQ.ID.NO.2 and SEQ.ID.NO.7) to amplify positive sample DNA respectively , Insert the amplified fragment into the T vector and verify it by sequencing, then quantitatively measure the OD260 / 280 value with a spectrophotometer, and then dilute the amplified plasmid to 10 with deionized water or TEBuffer 1 -10 10 Copy / ul as standard. Standards with different concentrations were detected by LAMP, and the reaction system and reaction conditions were the same as in Example 1.
[0073] After the LAMP reaction, use a PCR instrument or a temperature-controlled metal bath to control the temperature at 4°C, 65°C, and 95°C, respectively, and irradiate the reaction tube with a hand-held ultraviolet lamp to observe the fluorescence. At this time, the negative tube shows a change from no fluorescence to weak fluorescence to strong fluo...
Embodiment 3
[0075] Embodiment 3 The specific detection of the detection method of embodiment 1
[0076] Select DNA samples that have been clinically determined by PCR-reverse dot hybridization to determine the subtype of HPV infection, including 16, 18, 52, 68, and 33 positive samples, and use Axygen’s bacterial genome DNA extraction kit to extract the DNA of the samples to be tested. 20 ng of DNA (about 3000 copies of human genomic DNA) from each sample to be tested was taken as a template for LAMP detection, and the reaction system and reaction conditions were the same as in Example 1.
[0077] Comparison method: select DNA samples that have been confirmed by PCR-reverse dot hybridization in clinical practice, including positive samples of types 16, 18, 52, 68, and 33, and use Axygen’s bacterial genomic DNA extraction kit to extract samples to be tested Take 20ng of the DNA of the sample to be tested (about 3000 copies of human genomic DNA) as a template for LAMP detection. The reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com