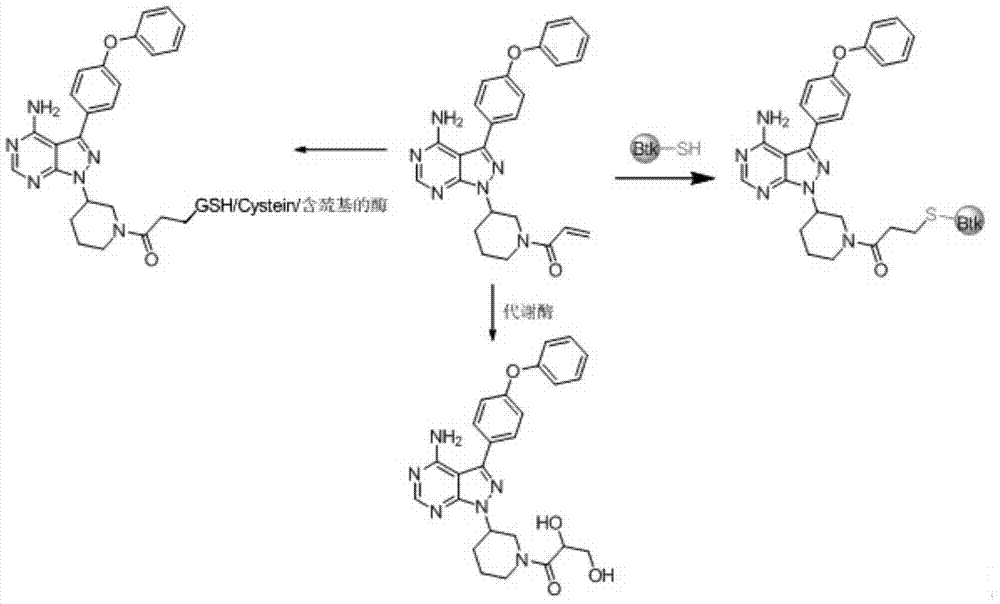
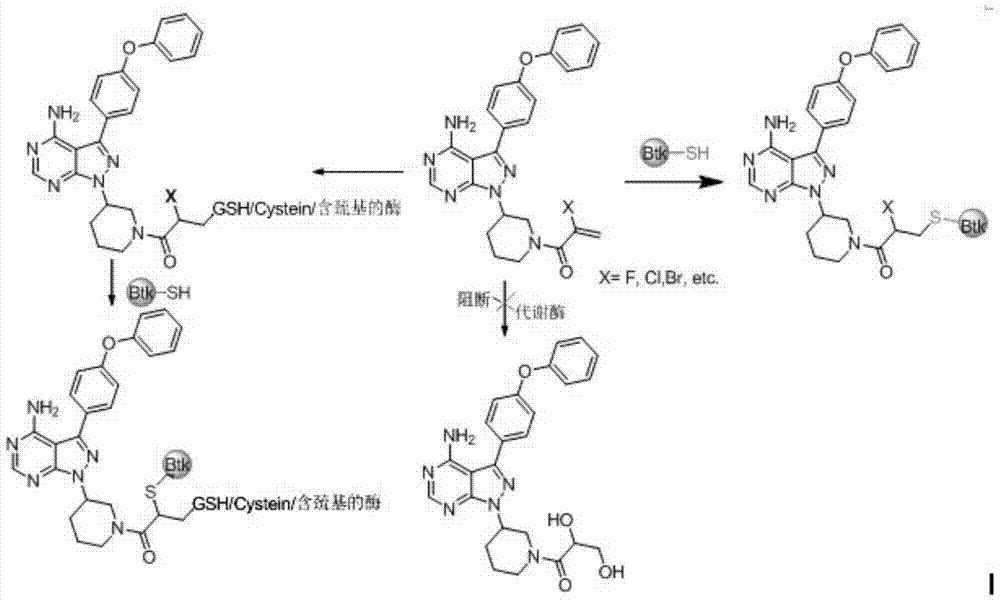
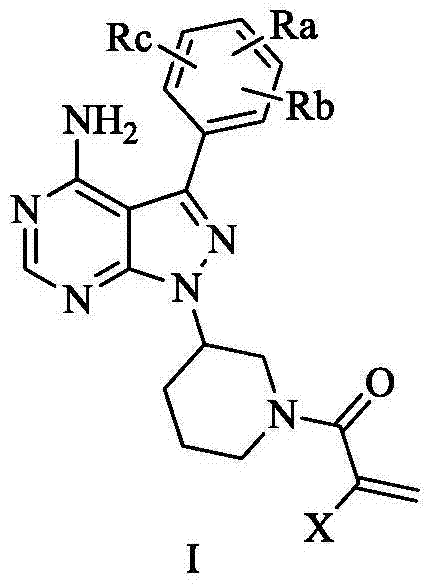
Double-site irreversible Brutons tyrosine kinase inhibitor
A tyrosine kinase and inhibitor technology, applied in the field of dual-site irreversible Bruton's tyrosine kinase inhibitor, anti-tumor application, which can solve the problems of affecting drug efficacy and increasing patient burden.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1. Preparation of key intermediate 4a
[0047]
[0048] Step 1. Synthesis of 3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (compound 2a)
[0049] 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (Compound 1) (2.61g, 10mmol), 4-phenoxyphenylboronic acid (3.85g, 18mmol) and potassium phosphate ( 5.375g, 25mmol) were added to the single-necked bottle successively, 1,4-dioxane (40mL) and water (10mL) were added, and under nitrogen protection, triphenylphosphopalladium (1.76g, 1.5mmol) was added, and reflux reaction 24h. After the reaction was completed, cool to room temperature, stir overnight, a yellow precipitate precipitated out, suction filtered, washed with water (50ml*3), and dried for 24 hours to obtain 2.18g of a yellow solid, which was 3-(4-phenoxyphenyl)-1H-pyrazole And[3,4-d]pyrimidin-4-amine (compound 2a), yield 72%.
[0050] Step 2. 3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine-1-carboxylic acid tert-butyl ester...
Embodiment 2
[0054] Embodiment 2. Preparation of key intermediate 4b
[0055]
[0056] Step 1. Synthesis of 3-(4-(4-methoxyphenoxy)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (compound 2b)
[0057] Synthetic steps refer to Example 1 step 1. Using a synthetic method similar to compound 2a, compound 2b (2.56g, yield 77% was prepared from 4-(4-methoxyphenoxy)phenylboronic acid (4.39g, 18mmol) ).
[0058] Step 2. 3-(4-Amino-3-(4-(4-methoxyphenoxy)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine- Synthesis of tert-butyl 1-formate (compound 3b)
[0059] Synthetic procedures refer to step 2 of Example 1. Compound 3b (1.26 g, yield 35%) was prepared by a method similar to that of compound 3a.
[0060] Step 3. 3-(4-(4-methoxyphenoxy)phenyl)-1-(piperidin-3-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine Synthesis of hydrochloride (compound 4b)
[0061] For the synthetic procedure, refer to step 3 of Example 1. Compound 4b was prepared by a method similar to that of compound 4a (0.59, 65% yield, [...
Embodiment 3
[0062] Embodiment 3. Preparation of key intermediate 4c
[0063]
[0064] Step 1. Synthesis of 3-(4-(3-methoxyphenoxy)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (compound 2c)
[0065] Synthetic steps refer to Example 1 Step 1. Using a synthetic method similar to compound 2a, compound 2c (2.49g, yield 75% was prepared from 4-(3-methoxyphenoxy)phenylboronic acid (4.39g, 18mmol) ).
[0066] Step 2. 3-(4-Amino-3-(4-(3-methoxyphenoxy)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine- Synthesis of 1-tert-butyl formate (compound 3c)
[0067] Synthetic procedures refer to step 2 of Example 1. Compound 3c (1.25 g, yield 35%) was prepared by a method similar to that of compound 3a.
[0068] Step 3. 3-(4-(3-methoxyphenoxy)phenyl)-1-(piperidin-3-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine Synthesis of hydrochloride (compound 4c)
[0069] For the synthetic procedure, refer to Step 3 of Example 1. Compound 4c (0.61 g, yield 68%, [M+H]=453.3) was prepared by a method similar to that o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com