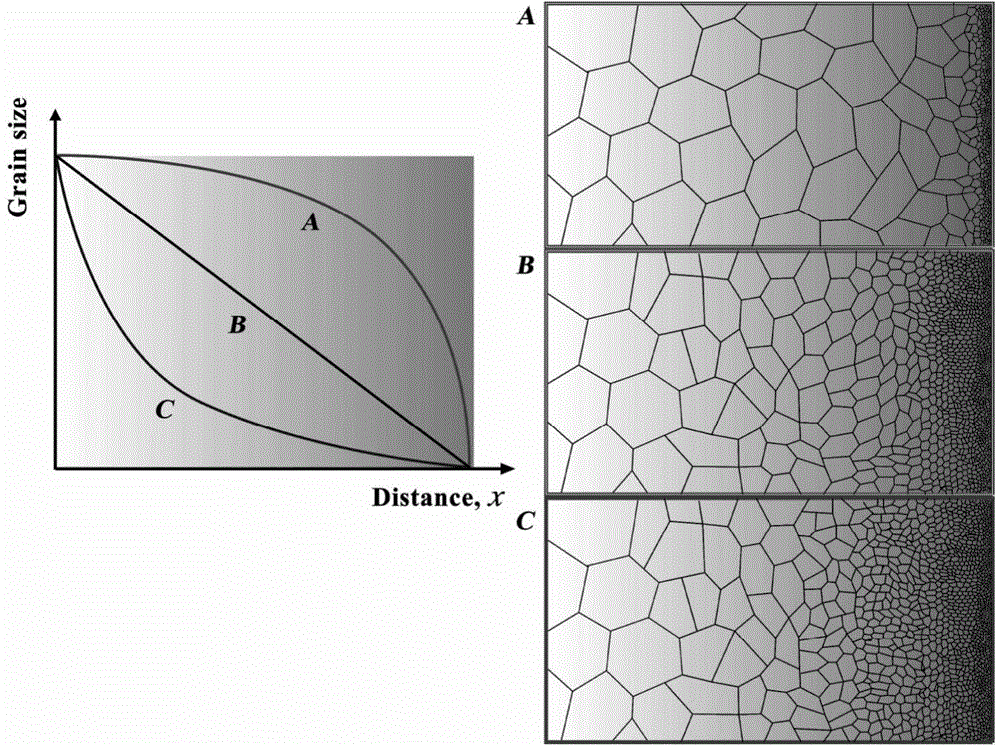
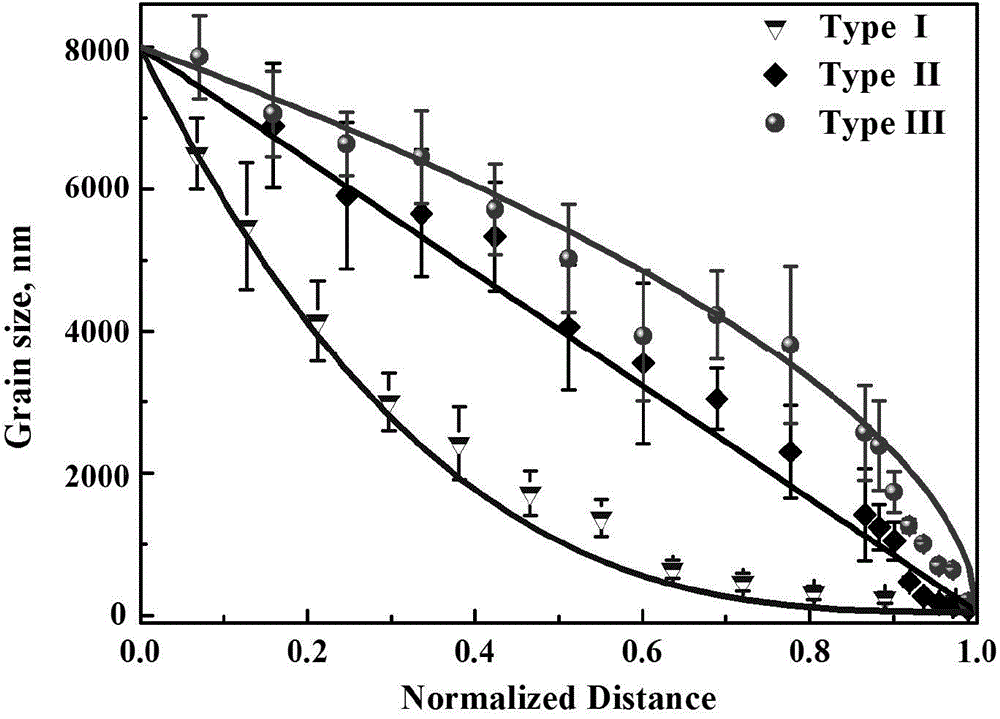
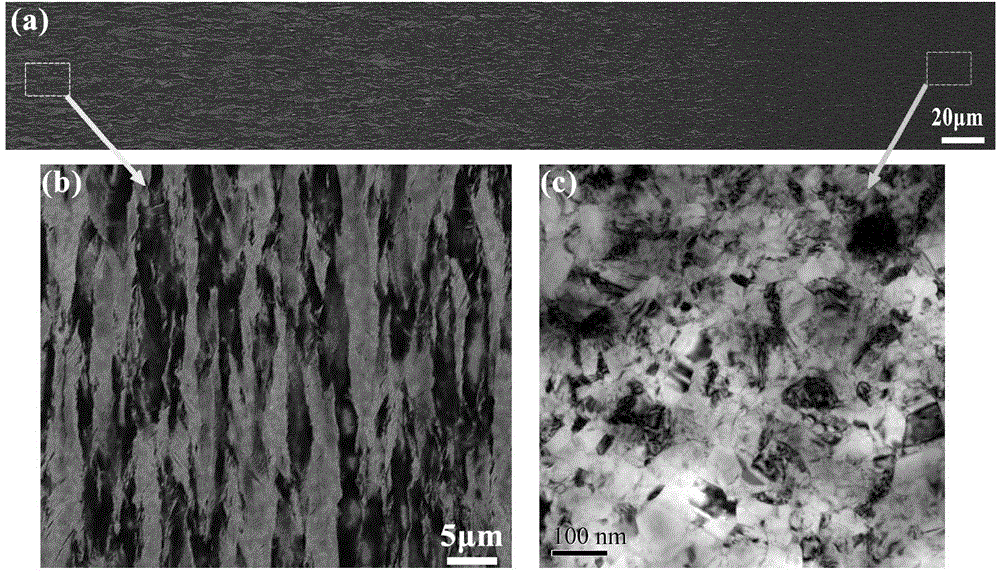
Crystalline grain scale gradient metallic nickel and controllable preparation method thereof
A technology of metal nickel and grain size, applied in the direction of cells, electrolytic process, electrolytic components, etc., can solve the problem of non-interface existence and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Use nickel as the consumable anode and titanium cathode, and configure 800ml of plating solution as required. The composition of the plating solution is:
[0021] NiSO 4 ·7H 2 O:300g / L, NiCl 2 ·6H 2 O: 45g / L, H 3 BO 3 :40g / L, C 12 h 25 OSO 2 Na: 0.05g / L. Weigh the above drugs with a balance and dissolve them in a beaker so that the volume of the solution is no more than three-quarters of the rated volume of the beaker. Stir the prepared plating solution with a magnetic stirrer and adjust the pH value to 4±0.2 with sodium hydroxide solution. The bath temperature is controlled at 55±1°C.
[0022] Computer controlled current density and additive concentration:
[0023] Current density: (1), the current density remains constant at 10mA / cm within the first 47.5h 2 , (2), after 0.75h the current density is changed from 10mA / cm 2 Gradually increase to 20mA / cm 2 , (3), and then within 0.75h the current density gradually increased to 30mA / cm 2 , (4), continue to gr...
Embodiment 2
[0027] Use nickel as the consumable anode and titanium as the cathode, configure 800ml of plating solution as required, and the composition of the plating solution is:
[0028] NiSO 4 ·7H 2 O:300g / L, NiCl 2 ·6H 2 O: 45g / L, H 3 BO 3 :40g / L, C 12 h 25 OSO 2 Na: 0.05g / L. Weigh the above drugs with a balance and dissolve them in a beaker so that the volume of the solution is no more than three-quarters of the rated volume of the beaker. Stir the prepared plating solution with a magnetic stirrer and adjust the pH value to 4±0.2 with sodium hydroxide solution. The bath temperature is controlled at 55±1°C.
[0029] Computer controlled current density and additive concentration:
[0030] The control current density is mainly divided into six stages: (1), the current density is kept constant at 5mA / cm in the first 25h 2 , (2), within 3h after that, the current density changed from 5mA / cm 2 Gradually increase to 20mA / cm 2 , (3), and then gradually increased to 30mA / cm withi...
Embodiment 3
[0034] With nickel as the consumption anode and steel as the cathode, configure 800ml of plating solution as required. The composition of the plating solution is:
[0035] NiSO 4 ·7H 2 O:300g / L, NiCl 2 ·6H 2 O: 45g / L, H 3 BO 3 :40g / L, C 12 h25 OSO 2 Na: 0.06g / L. Weigh the above drugs with a balance and dissolve them in a beaker so that the volume of the solution is no more than three-quarters of the rated volume of the beaker. Stir the prepared plating solution with a magnetic stirrer and adjust the pH value to 4±0.2 with sodium hydroxide solution. The bath temperature is controlled at 55±1°C.
[0036] Computer controlled current density and additive concentration:
[0037] Current density: (1), first keep the current density constant at 7mA / cm for 18h 2 , (2), and then within 3h the current density is changed from 7mA / cm 2 Gradually increase to 20mA / cm 2 , (3), the current density gradually increased to 30mA / cm in the following 3h 2 , (4), continue to gradually i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grain size | aaaaa | aaaaa |
| Grain size | aaaaa | aaaaa |
| Grain size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com