New route for synthesizing tulathromycin
A telamectin and synthetic route technology, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of high cost and safety, harsh reaction conditions, and difficult separation and purification of products, and achieve Easy to obtain, simple reaction process, easy to achieve effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
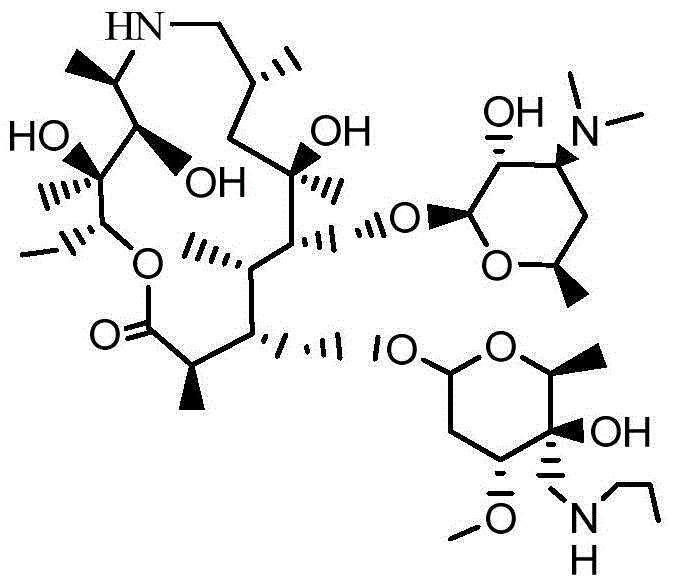
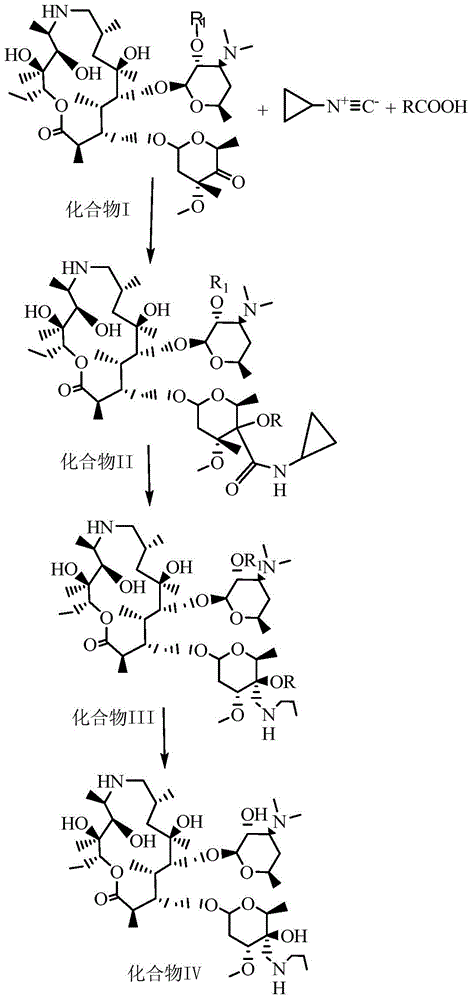
[0046] 2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[[2,6-dideoxy-3-C-methyl-3-O-methyl-4-oxo -α-L-nucleo-hexapyranosyl]-oxy]-2-ethyl-3,4,10-trihydroxy acid-3,5,8,10,12,14-hexamethyl-11- [[3,4,6-trideoxy-3-(dimethylamino)-2-O-acetyl-β-D-xyl-hexapyranosyl]oxy]-1-oxo-6-aza Cyclopentadecane (15-membered macrocycle) (with figure 2 Compound I) Preparation:
[0047] Add 140 g of hydroxyl-protected demethylazithromycin to a 2000 mL three-neck reaction flask, add 1400 ml of dichloromethane and stir to dissolve, heat up to 30°C, then add 71.7 g of pyridinium dichromate, keep stirring for 2 hours to obtain a reaction solution. After the reaction, add 400mL saturated NaCl solution to the above reaction solution, wash, and separate the phases, take the organic phase, add an appropriate amount of anhydrous sodium sulfate solid, filter, concentrate and dry to obtain compound I, white solid powder 114.4g, molar yield The rate is 82.2%, and the content is 92.3%.
Embodiment 2
[0049] 2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[[2,6-dideoxy-3-C-methyl-3-O-methyl-4-oxo -α-L-nucleo-hexapyranosyl]-oxy]-2-ethyl-3,4,10-trihydroxy acid-3,5,8,10,12,14-hexamethyl-11- [[3,4,6-trideoxy-3-(dimethylamino)-2-O-acetyl-β-D-xyl-hexapyranosyl]oxy]-1-oxo-6-aza Cyclopentadecane (15-membered macrocycle) (with figure 2 Compound I) Preparation:
[0050] Add 70g of hydroxyl-protected demethylazithromycin to a 1000mL three-necked reaction flask, add 700ml of dichloromethane and stir to dissolve, heat up to 30°C, then add 38.6g of chlorpyridinium chromate, and stir for 5 hours to obtain a reaction solution. After the reaction, add 200mL saturated NaCl solution to the above reaction solution, wash, and separate the phases, take the organic phase, add an appropriate amount of anhydrous sodium sulfate solid, filter, concentrate and dry to obtain compound I, white solid powder 56.3g, molar yield The rate is 80.1%, and the content is 91.3%.
[0051] Chromatographic conditions of...
Embodiment 3
[0053] (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[[2,6-dideoxy-3-C-methyl-3-O-methyl-4-C -[(cyclopropyl)amido]-4-O-carboethoxy-α-L-nucleo-hexapyranosyl]-oxy]2-ethyl-3,4,10-trihydroxy-3, 5,8,10,12,14-Hexamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-2-O-acetyl-β-D-wood-hexypyr Preparation of pyranosyl]oxy]-1-oxa-6-azacyclopentadecane (15-membered macrocycle) (compound II):
[0054] Add 500ml of ethyl acetate into a 1000mL three-necked reaction flask, raise the temperature to 20°C, then add 5.1g of cyclopropylnitrile and 4.6g of acetic acid, and stir evenly. The temperature was raised to 10° C., then 50 g of the product (Compound I) in Example 1 was added, and stirred for 2 hours to obtain a reaction solution. After the reaction, add 200mL saturated NaCl solution to the above reaction solution, wash and separate the phases, take the organic phase, add an appropriate amount of anhydrous sodium sulfate solid, filter, concentrate and dry to obtain compound II, 49.8g of white solid p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com