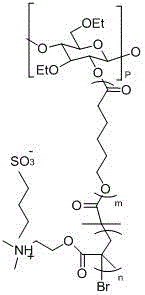
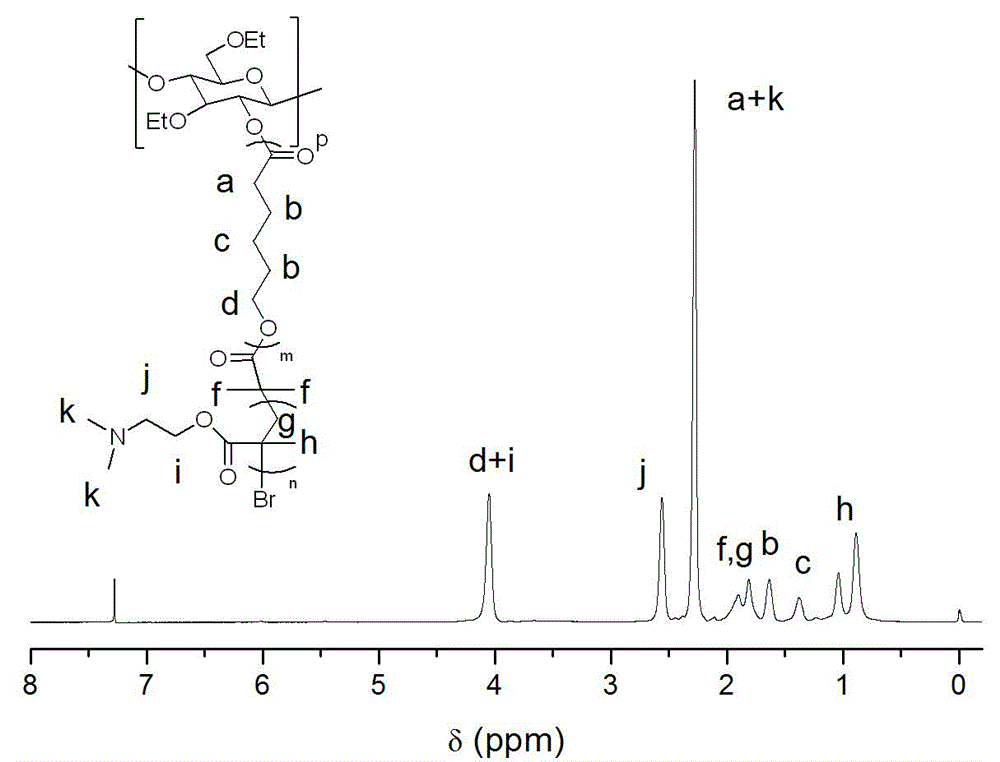
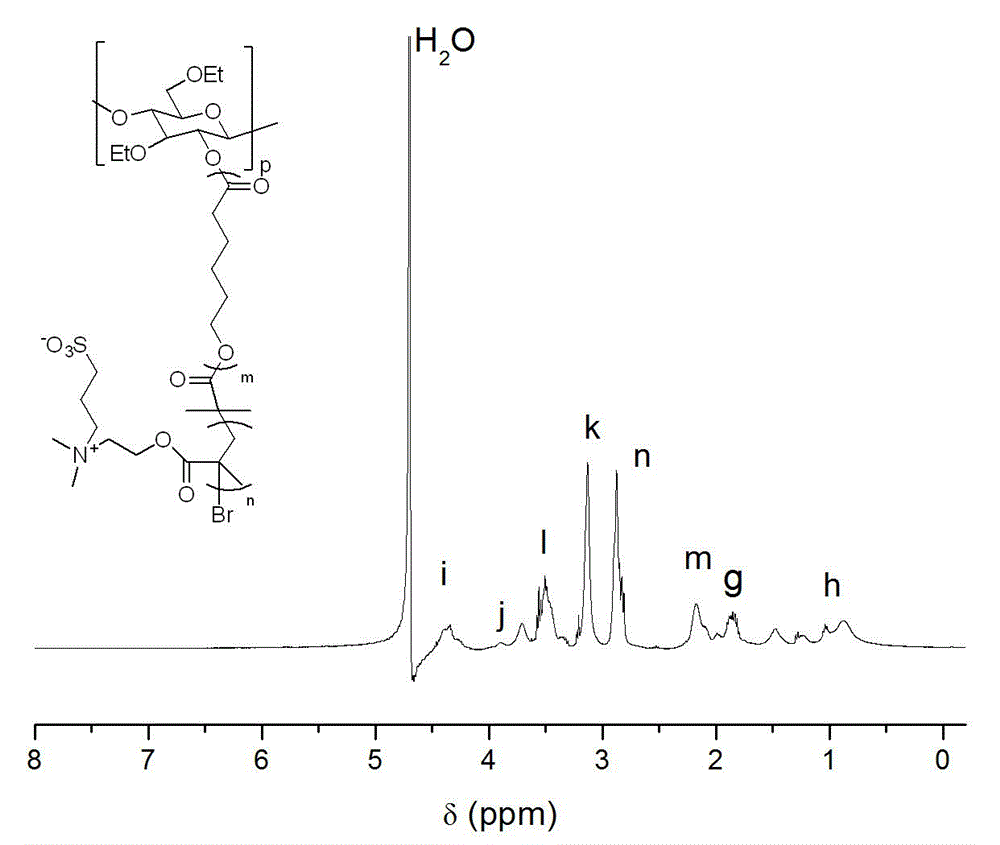
Preparation method of ethyl cellulose nano micelle with UCST (upper critical solution temperature)
A technology of ethyl cellulose and nano micelles, which is applied in the fields of polymer materials and biomedical engineering, can solve problems such as single function and poor solubility, and achieve the effects of wide sources and simple and easy synthesis methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 1.214 grams of ethyl cellulose in a round-bottomed flask, disperse and dissolve it with fresh anhydrous xylene, add 12.16 grams of ε-caprolactone, and then add 132 μL of stannous isooctanoate, and vacuum-fill with nitrogen 3 times, and reacted in an oil bath at 60°C for 12 hours under the protection of nitrogen, dissolved the obtained product in an appropriate amount of dichloromethane, then precipitated, filtered with suction, and dried in vacuum to obtain a macromolecular polymer whose main chain is ethyl cellulose substance (EC- g -PCL). Weigh the macromolecular polymer (EC- g -PCL) 3 g was dissolved in dichloromethane distilled to remove water, added 0.5 g of triethylamine distilled to remove water, the mixture was placed in an ice-water bath, 1.14 g of 2-bromoisobutyryl bromide was added dropwise to the mixture, and After that, the reaction was stirred at room temperature for 24h, and the products were respectively washed with saturated NaHCO 3 Aqueous sol...
Embodiment 2
[0032] Weigh 1.214 grams of ethyl cellulose in a round-bottomed flask, disperse and dissolve it with newly prepared anhydrous toluene, add 18.24 grams of ε-caprolactone, and then add 140 μl of stannous octoate. And under the protection of nitrogen, it was reacted in an oil bath at 80°C for 24 hours, and the obtained product was dissolved in an appropriate amount of chloroform, and then precipitated, filtered by suction, and dried in vacuum to obtain a macromolecular polymer (EC - g -PCL). Weigh the macromolecular polymer (EC- g -PCL) 3 g was dissolved in dichloromethane distilled to remove water, added 0.5 g of triethylamine distilled to remove water, the mixture was placed in an ice-water bath, 1.14 g of 2-bromoisobutyryl bromide was added dropwise to the mixture, and After that, the reaction was stirred at room temperature for 24h, and the products were respectively washed with saturated NaHCO 3 Aqueous solution and distilled water washing, carry out concentration again, ...
Embodiment 3
[0034] Weigh 1.214 grams of ethyl cellulose in a round-bottomed flask, disperse and dissolve it with newly prepared anhydrous dimethyl sulfoxide, add 12.16 grams of ε-caprolactone, and then add 156 μL of hydrogen chloride ether solution, and vacuum-fill Nitrogen process was carried out 3 times, and reacted in an oil bath at 100°C for 32 hours under the protection of nitrogen. The obtained product was dissolved in an appropriate amount of 1,2-dichloroethane, and then precipitated, filtered by suction, and dried in vacuum to obtain the main chain as B Cellulose-based macromolecular polymers (EC- g -PCL). Weigh the macromolecular polymer (EC- g -PCL) 3 g was dissolved in dichloromethane distilled to remove water, added 0.5 g of triethylamine distilled to remove water, the mixture was placed in an ice-water bath, 1.14 g of 2-bromoisobutyryl bromide was added dropwise to the mixture, and After that, the reaction was stirred at room temperature for 24h, and the products were respe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com