Vaccine for simultaneously preventing neospora and toxoplasma infection, preparation method and application thereof
A technology of Neospora and Toxoplasma gondii, which is applied in the field of parasitic disease vaccine genetic engineering, can solve the problem that the recombinant adenovirus vector vaccine for simultaneous prevention of Neospora and Toxoplasma gondii, the low protection rate, the inability to use neosporosis, etc. problem, to achieve the effect of good immune protection, high transgenic efficiency and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
[0050] In the vaccine of the present invention for simultaneously preventing the infection of Neospora and Toxoplasma, the amino acid sequence of the vaccine antigen is:
[0051] MSKIKASTELGKCAEFAYKTTAMDKSNQATQYRYPFVYDSKRRLCYILSVSMQRMEGSKYCSTNDDPVNLTWYCFEPQKSPTANHNLIFGSAYVGKDPDAFLTKCPNQALKGYRFGHWTNGRCHDYTELADSWIEAVDSKAQCWVKTFTNDEVASDKGHSCALGDQSHGDQPRGDSHGDHSGFDQPHWWNDKG
[0052] MSKIKASTDLGRCAEFAFKTVAMDKNNKATKYRYPFVYDSKKRLCHILYVSMQLMEGKKYCSVKGEPPDLTWYCFKPRKSVTENHHLIYGSAYVGENPDAFISKCPNQALRGYRFGVWKKGRCLDYTELTDTVIERVESKAQCWVKTFENDGVAQPHDTGLVDSGVDTGLVIDQVDTGLVIDQCWDTG
[0053] The nucleotide sequence encoding the above vaccine antigen is:
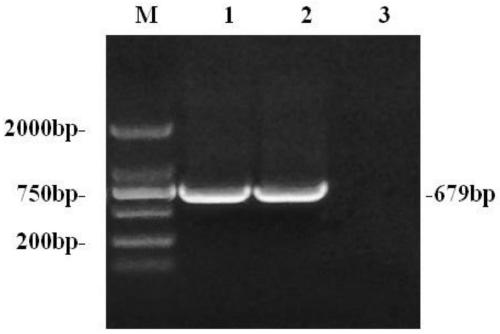
[0054] CCGCTCGAGGCCACCATGAGCAAAATCAAGGCGAGTACAGAGCTTGGGAAGTGCGCCGAATTCGCCTACAAGACGACTGCTATGGATAAAAGCAATCAGGCGACTCAGTACCGCTATCCATTTGTGTATGACTCTAAGCGGCGGCTGTGTTACATCCTTTCCGTATCGATGCAGCGAATGGAGGGCAGTAAGTACTGTTCTACCAACGATGACCCGGTTAATCTCACATGGTATTGCTTCGAGCCCCAAAAGAGTCCTACGGCGAATCATAATCTCATCTTCGGATCGGCCTACGTTGGAAAAGACCCAGATGCGTTCCTCACTAAATGCCCAAACCAAGCTCTTAAG...
no. 1 example
[0070] Animal immunity test of the recombinant adenovirus vector vaccine prepared in the first embodiment of the present invention
[0071] With BALB / c mice as experimental animals, 60 BALB / c mice were randomly divided into 3 groups, each with 20 mice, namely Ad5-Nc / Tg AMA1 vaccine group, pVAX1-Nc / TgAMA1 plasmid group and PBS control The group received intramuscular injection every 2 weeks for a total of 3 injections. Among them, pVAX1-Nc / TgAMA1 refers to the subcloning of Nc / TgAMA1 gene into pVAX1 eukaryotic expression vector. pVAX1-Nc / TgAMA1 was constructed and stored by the Laboratory of Preventive Veterinary Medicine of Yanbian University. The immune effect of the vaccine was compared. Ad5-Nc / TgAMA1 vaccine group one immunization 6×10 9 TCID 50 , 1.2×10 for the second and third immunity respectively 10 TCID 50 The pVAX1-Nc / TgAMA1 plasmid group was vaccinated with 0.1mg, 0.2mg for the second and third immunizations; PBS control group was vaccinated with 0.1mL for the first im...
no. 2 example
[0073] Neospora / Toxoplasma attack experiment
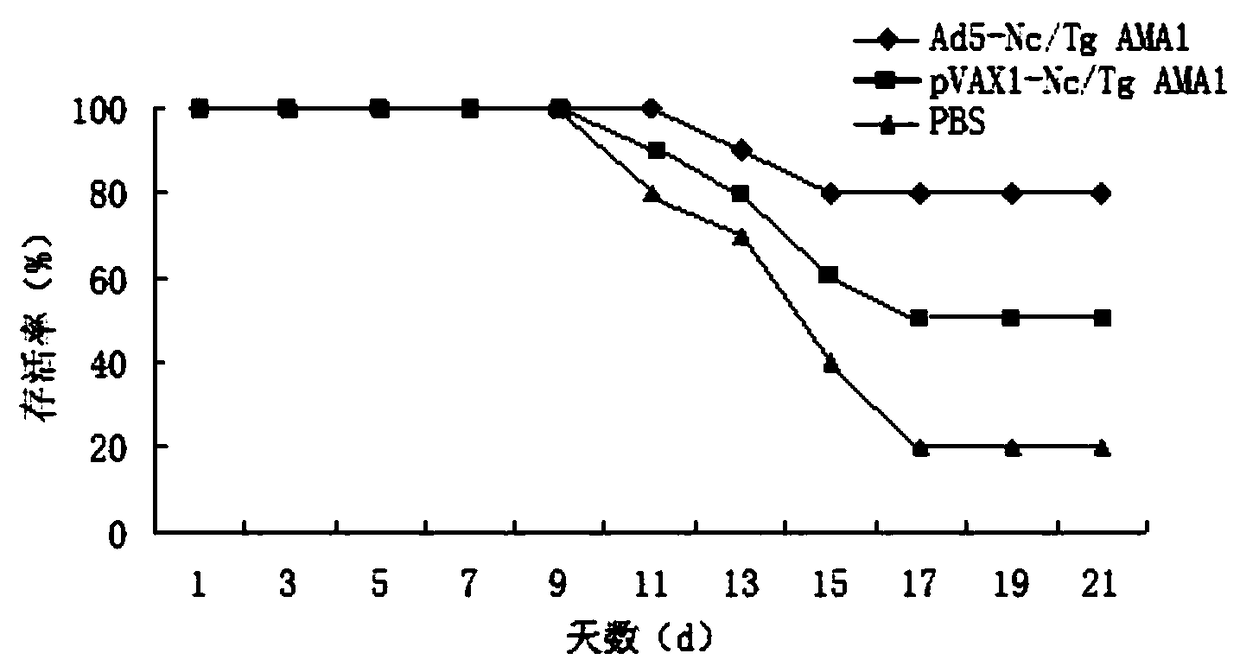
[0074] After three immunizations for 2 weeks, the 20 BABL / c mice in each group were divided into two groups, 10 in each group, and each in the abdominal cavity attacked the Neospora tachyzoites 10 6 Each of the two groups attacked the Toxoplasma RH strain tachyzoites 10 3 First, carry out the immune protection test of the vaccine, and observe the clinical symptoms and statistical survival rate of the mice. After 21 days of challenge experiment, BABL / c mice vaccinated with Ad5-Nc / TgAMA1 recombinant adenovirus vaccine had an 80% survival rate against Neospora tachyzoites, while the pVAX1-Nc / TgAMA1 plasmid group and PBS control group were respectively Survival rates are 50% and 20%; BABL / c mice vaccinated with Ad5-Nc / TgAMA1 recombinant adenovirus vaccine have 60% survival rates against Toxoplasma gondii, while the pVAX1-Nc / TgAMA1 plasmid group and PBS control group are respectively The survival rate is 20% and 0%.
[0075] The experiment...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com