Method for purifying or crystallizing influenza virus rna polymerase
A technology of RNA polymerase and influenza virus, applied in the biological field, can solve the problems of lack of influenza virus polymerase structure, structural biology research crystal structure obstacles, reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
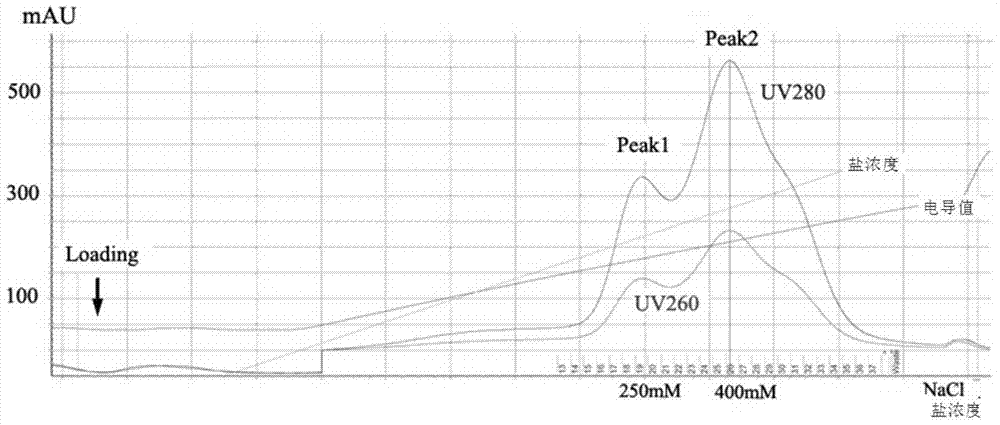
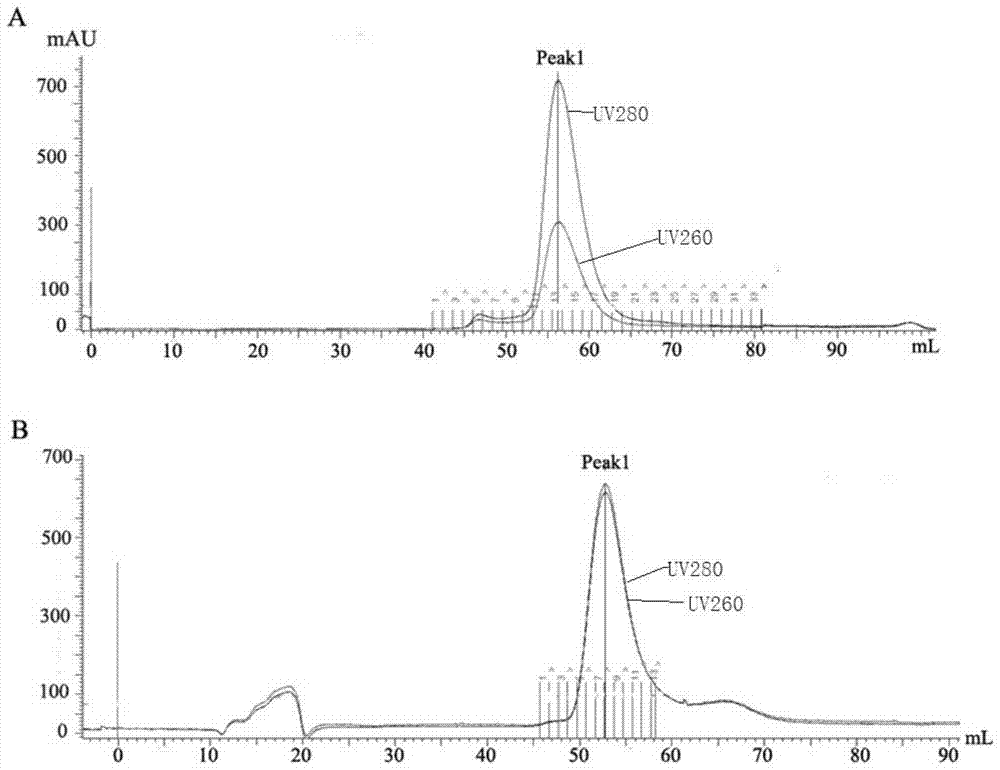
[0048] Embodiment 1, the crystallization of the influenza virus RNA polymerase that contains PB2-126 truncated body
[0049] The three subunits of the influenza virus RNA polymerase polymer precrystallized in this embodiment include PB1 protein, PA protein and PB2-126 truncated body;
[0050] The amino acid sequence of the PB2-126 truncated body is amino acid 1-126 from the N-terminal of sequence 2, and its coding gene is nucleotide 1-378 from the 5' end of sequence 5.
[0051] 1. Obtaining influenza virus RNA polymerase
[0052] 1. Expression of each subunit of influenza virus RNA polymerase
[0053] 1), the construction of the recombinant vector expressing each subunit of influenza virus RNA polymerase
[0054] Generally speaking, H5N1, WSN, PR8, 1918, and H3N2 gene DNAs were synthesized by gene synthesis methods according to the gene sequences published on the NCBI website. According to the respective sequences, DNA primers were designed respectively, and PCR amplificati...
Embodiment 2
[0142] Embodiment 2, the crystallization (comparative example) containing the influenza virus RNA polymerase of PB2-130 truncated body
[0143] The three subunits of the influenza virus RNA polymerase polymer precrystallized in this embodiment include PB1 protein, PA protein and PB2-130 truncated body;
[0144] The amino acid sequence of the PB2-130 truncated body is the 1st-130th amino acid from the 5' end of sequence 2, and the corresponding nucleotide sequence is the 1st-390th nucleotide from the 5' end of sequence 5.
[0145] 1. Obtaining influenza virus RNA polymerase
[0146] 1. Expression of each subunit of influenza virus RNA polymerase
[0147] 1), the construction of the recombinant vector expressing each subunit of influenza virus RNA polymerase
[0148] Due to the need for nickel column purification in the later stage, it is necessary to add a HIS tag to the carboxyl end of the subunit PB2-130 or PB1. Experiments were carried out on both of them. Any one of PB2-...
Embodiment 3
[0195] Embodiment 3, the crystallization (comparative example) containing the influenza virus RNA polymerase of PB2-103 truncated body
[0196] The three subunits of the influenza virus RNA polymerase polymer precrystallized in this embodiment include PB1 protein, PA protein and PB2-103 truncated body;
[0197] The amino acid sequence of the PB2-103 truncated body is the 1st-103th amino acid from the 5' end of the sequence 2, and the corresponding nucleotide sequence is the 1st-309th nucleotide from the 5' end of the sequence 5.
[0198] 1. Obtaining influenza virus RNA polymerase
[0199] 1. Expression of each subunit of influenza virus RNA polymerase
[0200] 1), the construction of the recombinant vector expressing each subunit of influenza virus RNA polymerase
[0201] Due to the need for nickel column purification in the later stage, it is necessary to add a HIS tag to the carboxyl end of the subunit PB2-130 or PB1. Experiments were carried out on both of them. Any one...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com