Pyrrolopyridazines as potassium ion channel inhibitors
A compound and enantiomer technology, applied in the field of pyrrolopyridazine compounds as potassium ion channel inhibitors, can solve the problems of neurotoxicity and gastrointestinal discomfort, and achieve the effect of reducing risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
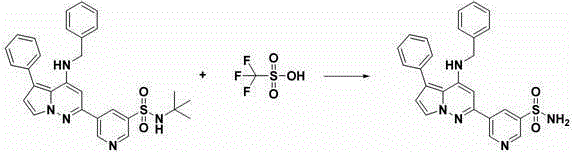
[0393] 5-(4-(Benzylamino)-5-phenylpyrrolo[1,2-b]pyridazin-2-yl)pyridine-3-sulfonamide
[0394]
[0395]
[0396] According to Fang et al., J. Med. Chem. , 53:7967-7978 (2010), with 2-methyl-1-pyrroline (0.831 g, 10.0 mmol, commercially available), NCS (10.7 g, 80.0 mmol) and NaOMe in MeOH (3M, 20 mL, 60.0 mmol) to synthesize commercially available methyl 3-chloro-1H-pyrrole-2-carboxylate (1.50 g, 94.0%, yellow solid). LCMS ( Condition 6 ): retention time 1.71 min, [M+1] = 160.10. 1 H NMR (400 MHz, CDCl 3 ) δ 3.90 (s, 3 H), 6.25 (t, J = 3.0 Hz, 1 H), 6.86 (t, J = 3.0 Hz, 1 H), 9.17 (br s, 1 H).
[0397] Synthesis of Monochloramine Reagent
[0398] Ammonium chloride (3.00 g, 56.1 mmol) was dissolved in diethyl ether (110 mL) and the solution was cooled to -5°C. Concentrated ammonium hydroxide (28 M, 4.70 mL, 120 mmol) was added dropwise. Commercial bleach (2 M, 72.0 mL, 0.144 mol) as sodium hypochlorite was added via the addition funnel over 15 min. The react...
Embodiment 2
[0418] N-((5-(4-(Benzylamino)-5-phenylpyrrolo[1,2-b]pyridazin-2-yl)pyridin-3-yl)sulfonyl)acetamide
[0419]
[0420]
[0421] CH 2 Cl 2(2 mL) to the suspension was added triethylamine (0.0140 mL, 0.0990 mmol), followed by acetyl chloride (0.00351 mL, 0.0490 mmol). The reaction mixture was stirred at ambient temperature for 12 h. The reaction mixture was quenched by adding water and washed with CH 2 Cl 2 (2 x 10 mL) extraction. The combined extracts were washed with anhydrous Na 2 SO 4 Dry, filter and concentrate under reduced pressure. The resulting residue was analyzed by preparative HPLC ( Condition 10, Purification as described in General Methods) gave N-((5-(4-(benzylamino)-5-phenylpyrrolo[1,2-b]pyridazin-2-yl)pyridine-3 as a yellow solid -yl)sulfonyl)acetamide (8.00 mg, 47.9%). LCMS ( Condition 2 ): retention time 1.89 min, [M+1] = 497.6. HPLC ( Condition 15 ): retention time 20.82 min, purity 98.40%. 1 H NMR (400 MHz, DMSO-d 6 ) δ 1.85 (s, 3 H), ...
Embodiment 3
[0423] N-(5-(4-(Benzylamino)-5-phenylpyrrolo[1,2-b]pyridazin-2-yl)pyridin-3-yl)acetamide
[0424]
[0425]
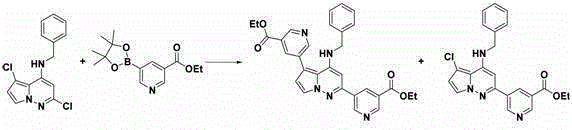
[0426] According to the method described in Example 1 for the preparation of 5-(4-(benzylamino)-5-chloropyrrolo[1,2-b]pyridazin-2-yl)-N-(tert-butyl)pyridine -3-sulfonamide method using N-benzyl-2,5-dichloropyrrolo[1,2-b]pyridazin-4-amine (150 mg, 0.513 mmol), 5-(4,4, 5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-amine (226 mg, 1.03 mmol, commercially available), Pd(TPP) 4 (59.3 mg, 0.0510 mmol) and Cs 2 CO 3 (502 mg, 1.54 mmol) prepared 2-(5-aminopyridin-3-yl)-N-benzyl-5-chloropyrrolo[1,2-b]pyridazin-4-amine (100 mg, 47.3 %, off-white solid). The residue was purified by combiflash (REDISEP®, silica gel, 12 g, 2% MeOH / DCM) and the product obtained was further purified by washing with diethyl ether. LCMS ( Condition 4 ): retention time 2.00 min, [M+1] = 350.0. 1 H NMR (400 MHz, DMSO-d 6 ) δ 4.67 (d, J = 6.0 Hz, 2 H), 5.45 (br s, 2 H), 6.04 (s, 1 H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com