Isoquinolinium compound, producing method and application of its salt
A technology of tetrahydroisoquinoline hydrochloride and compound is applied in the application field of preparing medicine for treating antiarrhythmic diseases, and can solve the problems of irregularity, poor oral absorption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
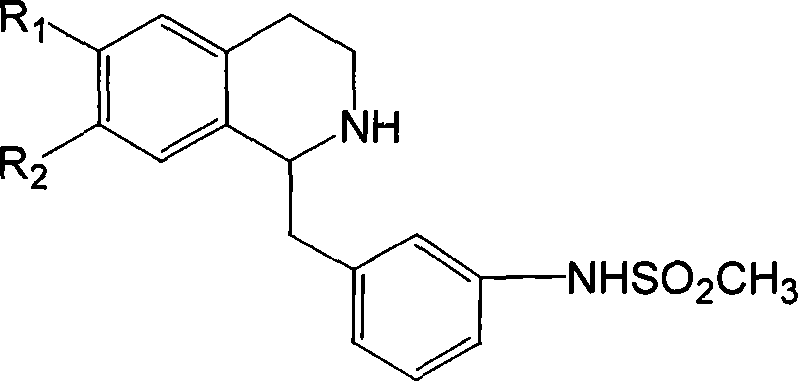
[0059] 1-(2,5-dimethoxybenzyl)-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline hydrochloride SIPI 926
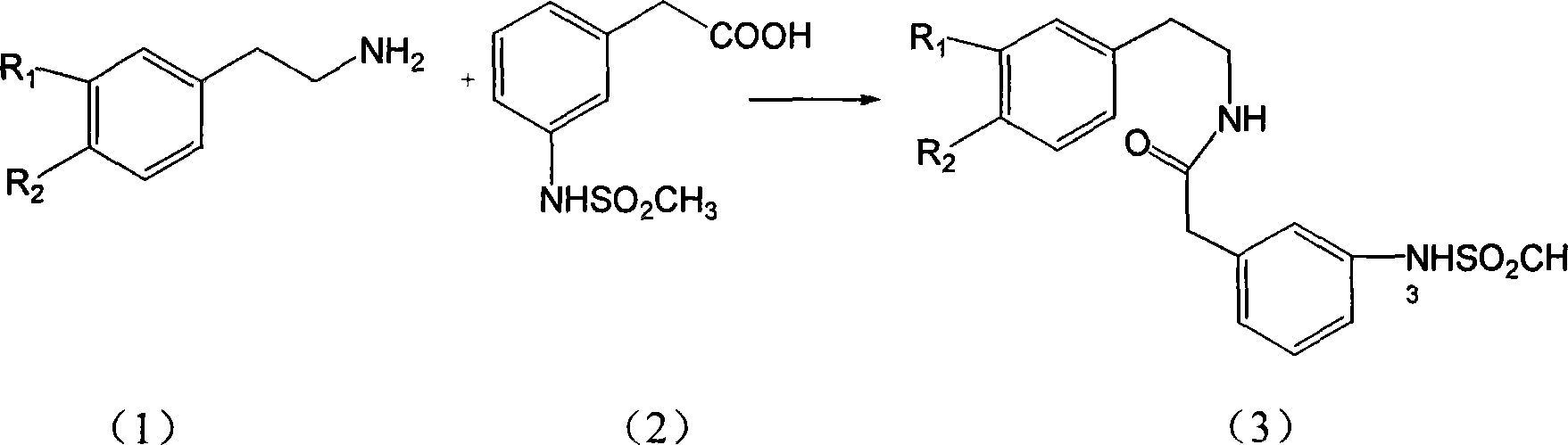
[0060] (1) Mix 16.3g (0.1mol) piperonylethylamine with 19.0g (0.1mol) 2.5-dimethoxyphenylacetic acid, heat at 170-180°C for 4 hours, cool, dissolve in chloroform, and then use 2NHCl, 2NNaOH Wash with water, dry over anhydrous magnesium sulfate, evaporate the solvent under reduced pressure, and the residue is washed with EtOH-H 2 O was recrystallized to obtain 22.1 g of N-(3,4-methylenedioxyphenethyl)-3,4-dimethoxyphenylacetamide with a yield of 65% and a melting point of 119-120°C.
[0061] Elemental Analysis C 19 h 21 NO 5 Calculated %: C 66.74, H 5.87, N 4.07
[0062] Found %: C 66.46, H 6.16, N 4.08
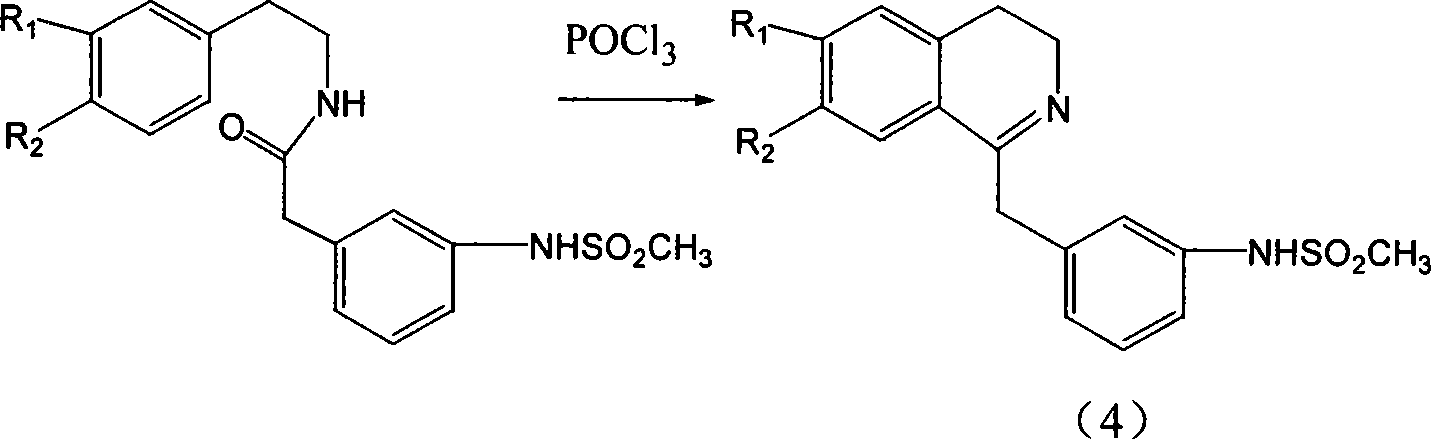
[0063] (2) Dissolve 17g (0.05mol) of the above amide in dry chloroform, add 26ml of phosphorus oxychloride, reflux for 4 hours, evaporate the solvent and excess phosphorus oxychloride under reduced pressure, and wash the residue with petroleum ether to obtain a solid ...
Embodiment 2
[0070] 1-(4-methoxybenzyl)-6-methoxy-7-benzyloxy-1,2,3,4-tetrahydroisoquinoline hydrochloride SIPI 1124
[0071] (1) Use 3-methoxy-4-benzyloxyphenethylamine and 4-methoxyphenylacetic acid as raw materials to prepare the corresponding amides according to Example 1 (1).
[0072] (2) take above-mentioned amide as raw material and make corresponding dihydroisoquinoline hydrochloride by the operation of embodiment 1 (2)
[0073] (3) Using the above-mentioned dihydroisoquinoline hydrochloride as a raw material, SIPI 1124 was prepared according to Example 1 (3), with a melting point of 176-177°C.
[0074] Elemental Analysis C 25 h 28 NO 3 Cl H 2 Calcd % O: C 67.63, H 6.81, N 3.16, Cl 7.99
[0075] Found %: C 67.93, H 7.00, N 2.85, Cl 8.19
Embodiment 3
[0077] 1-(3-Methanesulfonamidobenzyl)-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline hydrochloride C-391
[0078] (1) 8.5g (0.047mol) 3-nitrophenylacetic acid, 16ml SOCl 2 , mixed with 16ml benzene, heated to reflux for 4h, evaporated the solvent and excess SOCl under reduced pressure 2 In 3-nitrophenylacetyl chloride.
[0079] Dissolve 8g (0.04mol) of piperonylethylamine in 60ml of dichloroethane, and add 10% NaOH solution and 7.8g (0.039mol) of 3-nitrophenylacetyl chloride dropwise at 0-5°C. Alkanes solution, control PH8-9, add, stir at room temperature for 4h, precipitate solid, filter, wash with 5% HCl, wash with water, dry, recrystallize from ethanol to get 12.5g of N-(3,4-methylenedioxybenzene Ethyl)-3-nitrophenylacetamide, the yield is 86%, and the melting point is 128-130°C.
[0080] (2) 10g iron powder and 5% NH 4 Mix 50ml of Cl solution, stir at 100°C for 20 minutes, then cool to 60°C, add 10g (0.03mol) N-(3,4-methylenedioxyphenethyl)-3-nitrophenylacetamide an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com