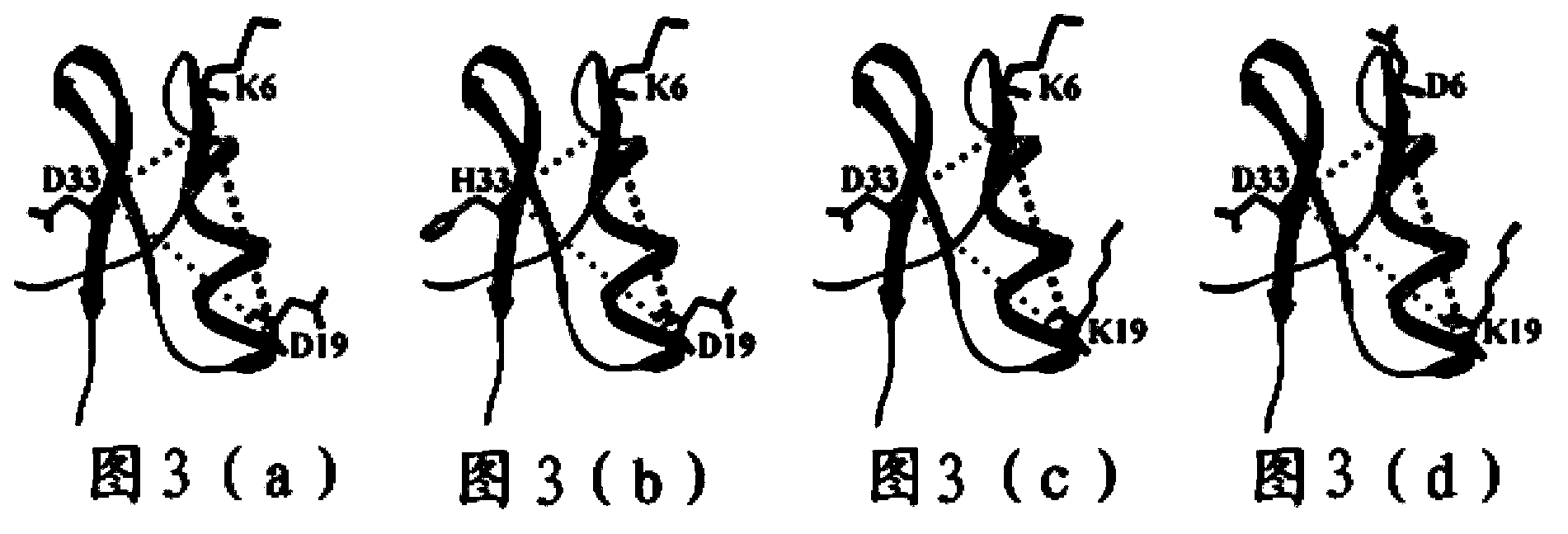
Molecular design of targeted potassium channel Kv1.3 active polypeptide and preparation and application thereof
A technology of DNA molecules, hypersensitivity, applied in the field of biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1: have the acquisition of the DNA molecule of nucleotide sequence as shown in SEQ ID NO:5~7
[0088] 1. Amplification primer design:
[0089] With the nucleotide sequence shown in SEQ ID NO:5 as the template sequence design amplification primer, the primer sequence used for amplifying the DNA molecule of the nucleotide sequence shown in SEQ ID NO:5 is as SEQ ID NO:8 ~11 shown;
[0090] With the nucleotide sequence shown in SEQ ID NO:6 as the template sequence design amplification primers, the primer sequence used for amplifying the DNA molecule of the nucleotide sequence shown in SEQ ID NO:6 is as SEQ ID NO:12 ~15 shown;
[0091] With the nucleotide sequence shown in SEQ ID NO:7 as the template sequence design amplification primers, the primer sequence used for amplifying the DNA molecule of the nucleotide sequence shown in SEQ ID NO:7 is as SEQ ID NO:16 ~19 shown.
[0092] In the process of designing the primers, add the enzyme cleavage sites required f...
Embodiment 2
[0106] Embodiment 2: The transformant construction comprising the nucleotide sequence DNA molecule shown in SEQ ID NO:5~7
[0107] Take the transformant construction process comprising the nucleotide sequence DNA molecule shown in SEQ ID NO: 5 as an example:
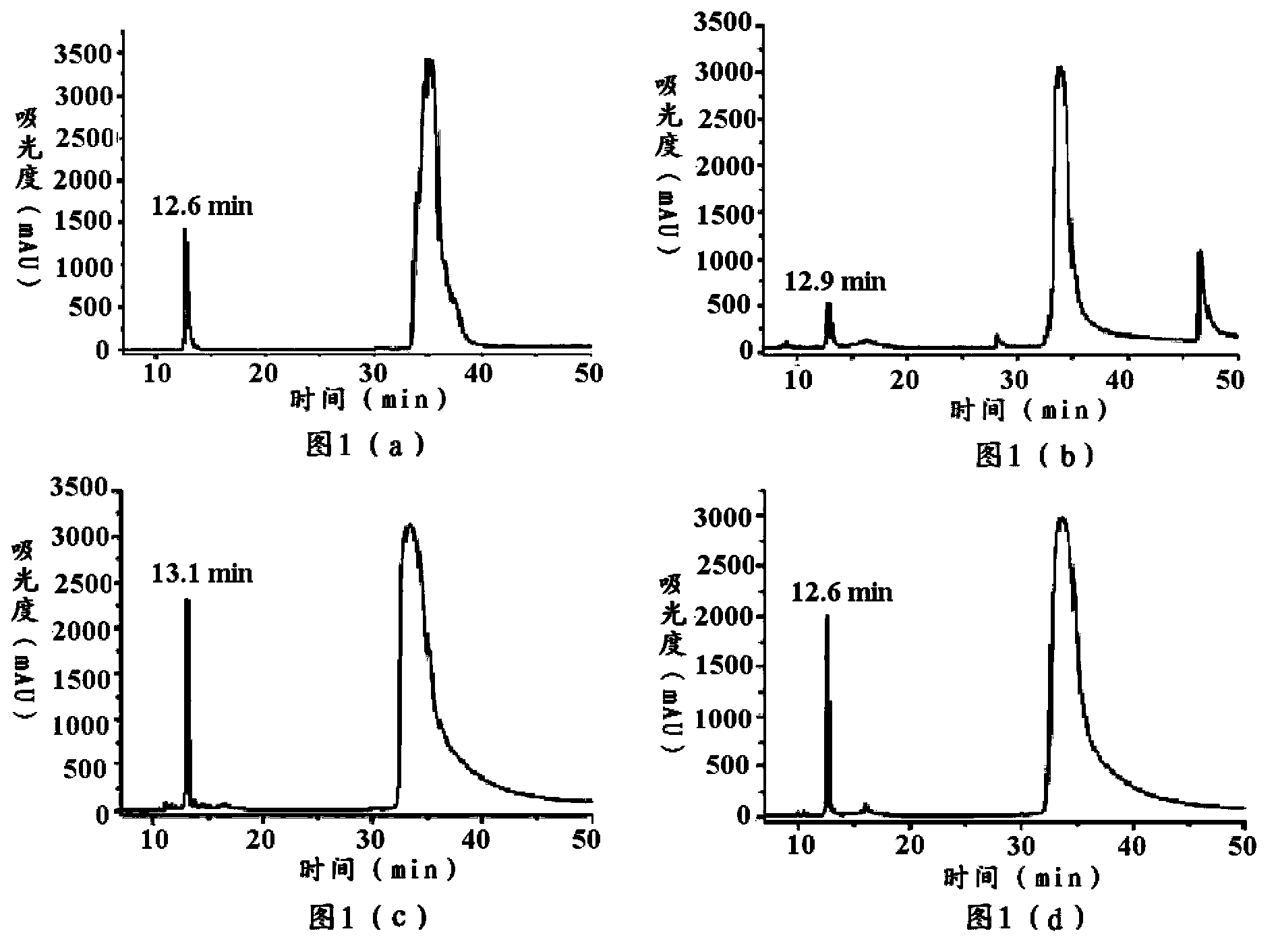
[0108] Carry out agarose gel electrophoresis to the PCR amplified product having the nucleotide sequence shown in SEQ ID NO:5, reclaim the target fragment that size is 111bp, obtain having the nucleotide sequence shown in SEQ ID NO:5 DNA molecule.
[0109] EcoR I and Xho I were used to perform double enzyme digestion on the recovered DNA molecule, gel electrophoresis and recovery to obtain a DNA molecule having a cohesive end with the nucleotide sequence shown in SEQ ID NO:5. The double enzyme digestion step was carried out according to the product manual. Using T4 ligase, it was ligated into the expression vector pGEX-6p-1 that had been cut with EcoR I and Xho I to obtain a recombinant vector including the nucleotide ...
Embodiment 3
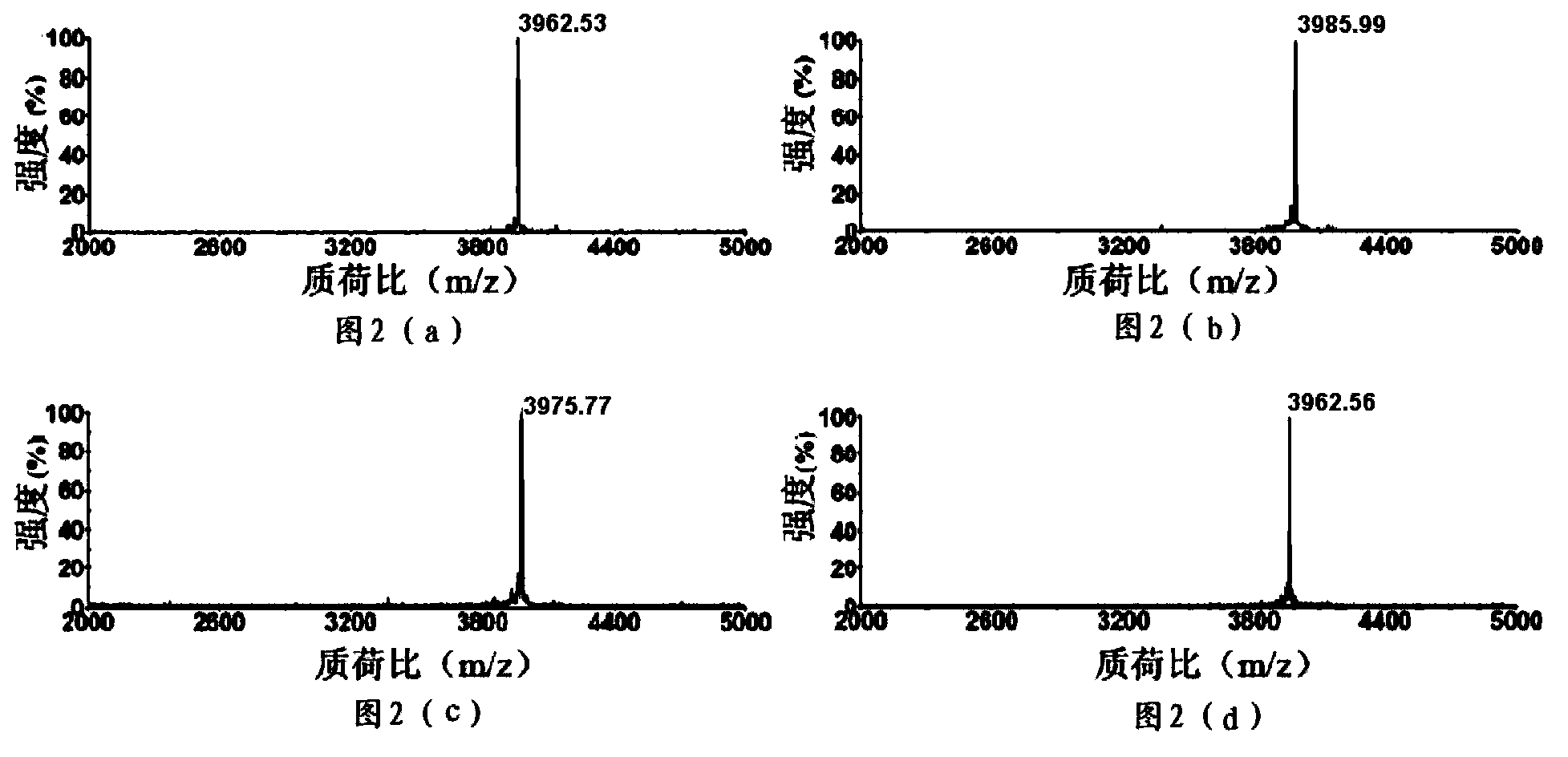
[0115] Example 3: Expression and purification of polypeptides having an amino acid sequence as shown in SEQ ID NO:2-4
[0116] Take the expression and purification of a polypeptide having an amino acid sequence as shown in SEQ ID NO: 2 as an example:
[0117] Inoculate the positive bacterial strain identified in Example 2 into 3 mL of LB medium containing 100 μg / mL ampicillin, and after culturing at 37°C for 12 hours, transfer the culture solution to 1 L of fresh LB medium containing 100 μg / mL ampicillin In the medium, after culturing at 37° C. for 4 hours, IPTG was added to make the final concentration 0.1 mM, and the expression was induced at 37° C. for 4 hours.
[0118] Collect the cells in the induced cell solution, suspend them in buffer (50 mM Tris-Cl, 1.0 mM EDTA, pH 8.0), disrupt the cells by ultrasonic, and collect the supernatant by centrifugation. The obtained supernatant was eluted by GST affinity chromatography to obtain a fusion protein solution, and the obtaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com